TABLE 3
Relative risk of hysterectomy and uterine hyperplasia during STAR
| Characteristic | Women who took tamoxifen | Women who took raloxifene | Relative risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy during study | 246 | 92 | 0.37 (0.28, 0.47) |
| Hyperplasia • with atypia • without atypia | 100 15 85 | 17 2 15 | 0.17 (0.09, 0.28) 0.13 (0.01, 0.56) 0.17 (0.09, 0.30) |
What is raloxifene’s effect on the heart?
The Raloxifene Use for The Heart (RUTH) trial explored the primary endpoints of coronary artery disease (CAD) and breast cancer in more than 10,000 women who had CAD or multiple risk factors for it.16 This study began prior to the Women’s Health Initiative, at a time when hormone replacement therapy was widely believed to reduce CAD.
In the double-blinded, randomized, placebo-controlled RUTH trial, raloxifene had no significant effect on primary coronary events (533 vs 553; hazard ratio [HR], 0.95; 95% CI, 0.84–1.07). Even in this population, however, there was a 44% reduction in invasive breast cancer (40 vs 70 events; HR, 0.56; 95% CI, 0.38–0.83).
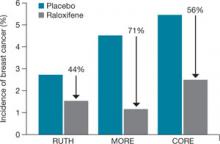
Based on these results, the FDA approved raloxifene for the “reduction in risk of invasive breast cancer in postmenopausal women at high risk for breast cancer,” as well as for the “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” ( FIGURE ).
FIGURE How raloxifene reduced invasive breast cancer in three trials
Raloxifene significantly reduced the risk of cancer, compared with placebo, in the Raloxifene Use for The Heart (RUTH), Multiple Outcomes of Raloxifene Evaluation (MORE), and Continuing Outcomes Relevant to Evista (CORE) trials.
CASE 2 RESOLVED
S. T. begins taking raloxifene 60 mg daily to lower her risk of invasive breast cancer. Although she temporarily experienced hot flashes after initiating the drug, they are only mildly bothersome, and she continues raloxifene therapy. She says she is grateful that there is an agent that can help her reduce the likelihood that she will develop breast cancer, and protection of her BMD is an added benefit.
CASE 3: At risk for both breast cancer and bone fracture
A. N., 63, is a nulliparous Caucasian woman who weighs 134 lb and stands 5 ft 4 in tall. She reached menarche when she was 12 years old and entered menopause at 49.
Although A. N. has never had a breast abnormality, her 59-year-old sister was just given a diagnosis of breast cancer. Her Gail score reveals that she has a 3.1% risk of developing breast cancer over the next 5 years.
In addition to her concerns about breast cancer, A. N. is worried about hip fracture—because her mother suffered one after menopause and because her T-score is –1.9 at the hip and –2.1 at the spine. A. N. has used steroids off and on for much of her life for asthma. Her FRAX score indicates that she has a 2.8% risk of hip fracture and a 25% risk of major osteoporotic fracture over the next 10 years.
What do you offer her?
Because of new FRAX criteria, this osteopenic woman is now a candidate for medication to reduce her risk of major osteoporotic fracture, and raloxifene is a good option. Her Gail score of 3.1% also makes her a good candidate for breast cancer risk reduction with raloxifene.
CASE 3 RESOLVED
Because A. N. needs an agent that benefits both breast and bone, you prescribe raloxifene. The drug should significantly reduce her risk of both invasive breast cancer and bone fracture, without increasing her risk of endometrial hyperplasia and cancer, both of which are associated with tamoxifen in her age group.
Aromatase inhibitors
A fairly new class of drugs being explored for their ability to reduce the risk of breast cancer is aromatase inhibitors. Substantial evidence suggests that estrogens facilitate the development of breast cancer in animals and in women, although the precise mechanism remains unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells and thereby increases the risk of genetic mutation that could lead to cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal women, the primary site of this action is in the ovary. In postmenopausal women, this conversion occurs primarily in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Aromatase inhibitors may be more effective than SERMs in preventing breast cancer because of their dual role: blocking both the initiation and promotion of breast cancer.18 These agents reduce levels of the genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. At the same time, aromatase inhibitors also block tumor promotion by lowering tissue levels of estrogen and preventing cell proliferation.