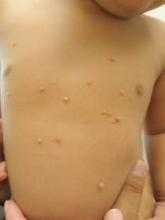
PARK CITY, UTAH – In her practice as a pediatric dermatologist, Dr. Sheryll L. Vanderhooft sees her share of children who present with molluscum contagiosum and warts. At the annual meeting of the Pacific Dermatologic Association, she shared her approach to caring for patients who present with these two common conditions.
The root cause of molluscum contagiosum is a poxvirus infection that spreads by skin-to-skin contact. It takes an average of 6 months to 2 years for the host immune response to occur. “During that time parents panic and some need a lot of hand-holding,” said Dr. Vanderhooft, professor of pediatric dermatology at the University of Utah School of Medicine, Salt Lake City. “They seek treatment primarily due to associated dermatitis triggered or exacerbated by the virus. There’s also the risk of spreading the condition to other children and the stigma of visible lesions.”
No FDA-approved therapies for molluscum contagiosum currently exist. “There are many options, but none of them are guaranteed to hasten resolution,” she said. “This is a very important point to deliver to parents before you start trying to do a lot of therapy.” Common treatment options are cryotherapy and cantharadin destruction, curettage, treatment with topical imiquimod or retinoids, oral cimetidine, and injections of Candida antigen.
According to two retrospective studies of Candida antigen injection, complete clearance occurred in 56% of patients after an average of 3 treatments, while partial clearance occurred in 28-38% of patients after an average of 4 treatments (Pediatr. Dermatol. 2008; 25:189-92 and Pediatr. Dermatol 2011; 28:254-8). The main side effect was local pain from the injections.
Antiviral and antitumor effects are seen with imiquimod cream through activation of the innate immunity and upregulation of cytokines, such as interferon-alpha, Dr. Vanderhooft said. It’s FDA approved for genital and perianal warts in patients older than 12 years of age, as well as for actinic keratosis, superficial basal cell carcinoma, and antiviral-resistant HSV in patients older than 12 years of age. In a prospective study, researchers compared imiquimod cream 5 times per week up to 16 weeks, with cryotherapy for the treatment of molluscum contagiosum in children (Pediatr. Dermatol. 2010; 27[4]:388-94). More than half of patients (60%) achieved clearance by week 6 and 92% achieved clearance by week 12. There were no relapses observed at six months. “Liquid nitrogen cleared the lesions faster, but caused more post-inflammatory dyspigmentation, and there were a few relapses in this group,” Dr. Vanderhooft said.
The downside to imiquimod treatment is that it requires prolonged use, it’s expensive, “and a lot of insurance companies will not pay for it because it’s not FDA approved for young children,” she said. “It can also cause irritant dermatitis, which is a difficult problem when you already have a kid who may be atopic and you’re trying to treat molluscum.” Also, according to the package insert for Aldara cream, imiquimod administered three times per week for 16 weeks was not shown to be superior to vehicle alone in two randomized, controlled trials that were completed in 2006 but never published. There were 323 children in one study, with complete clearance at 18 weeks in 24% of children treated with imiquimod, compared with 26% of children treated with vehicle alone. There were 379 in the second study with complete clearance in 24% and 28% of children, respectively.
Children who were treated with imiquimod in these two studies were more likely to experience application site reactions, otitis media, and conjunctivitis. In addition, a pharmacokinetic study of 22 children with molluscum involving at least 10% of body surface area showed systemically detectable drug levels after single and multiple doses 3 times a week for 4 weeks. The findings from these three studies led to changes in the FDA approved package insert in 2008. Section 1.4 of the “indications and usage” section of the package insert now reads: “Aldara cream has been evaluated in children ages 2012 years with molluscum contagiosum and these studies failed to demonstrate efficacy.” With that in mind, Dr. Vanderhooft’s recommendation “is to either stop prescribing it in children or prescribe it once daily or twice daily as tolerated, not every other day.”
While molluscum contagiosum is caused by a poxvirus, warts are growths on the skin caused by infection with human papillomavirus (HPV). On average, 75% of children will develop immunity to HPV within 3 years, “whether you do nothing, or whether you try to treat it,” she said. “Parents seek treatment when they are large, spreading, or causing social stigma. There are many treatment options, but none are guaranteed to hasten resolution. This needs to be driven home to patients when you’re counseling them.” Numerous treatment options exist, ranging from destruction with cryotherapy and pulsed-dye laser to salicylic acid and various topical or injectable agents. The only FDA-approved option is imiquimod cream, which is approved for genital and perianal warts only in patients 12 years of age and older.