STUDY SUMMARY
Spironolactone vs other drugs
The placebo-controlled crossover RCT conducted in the UK by Williams et al was the first to directly compare spironolactone with other medications for the treatment of resistant hypertension in adults already taking triple therapy.1The trial randomized 335 individuals with a mean age of 61.4 (range, 18 to 79), 69% of whom were male; 314 were included in the intention-to-treat analysis.1
Enrollment criteria for resistant hypertension specified a clinic-recorded SBP of ≥ 140 mm Hg (or ≥ 135 mm Hg in those with diabetes) and home SBP (in 18 readings over four days) of ≥ 130 mm Hg.1 To ensure fidelity to treatment protocols, the investigators directly observed therapy, took tablet counts, measured serum ACE activity, and assessed BP measurement technique, with all participants adhering to a minimum of three months on a maximally dosed triple regimen.
Among subjects, 14% had diabetes and 7.8% reported tobacco use. Average weight was 93.5 kg (205.7 lbs).1 Because of the expected inverse relationship between plasma renin and response to AAs, plasma renin was measured at baseline to test whether resistant hypertension was primarily due to sodium retention.1
Four 12-week rotations
All participants began the trial with four weeks of placebo, followed by randomization to 12-week rotations of once-daily oral treatment with (1) spironolactone 25 to 50 mg, (2) doxazosin modified release 4 to 8 mg, (3) bisoprolol 5 to 10 mg, and (4) placebo.1 Six weeks after initiation of each study medication, participants were titrated to the higher dose. There was no washout period between cycles.
The primary outcome was mean SBP measured at home on four consecutive days prior to the study visits in weeks 6 and 12. Participants were required to have at least six BP measurements per each six-week period in order to establish a valid average. Primary endpoints included the difference in home SBP between spironolactone and placebo, the difference in home SBP between spironolactone and the mean of the other two drugs, and the difference in home SBP between spironolactone and each of the other two drugs.
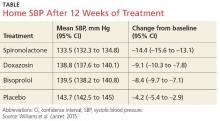
The results. Spironolactone lowered SBP more than placebo, doxazosin, and bisoprolol (see the Table).1 Clinic measurements were consistent with home BP readings.
Overall, 58% of participants achieved goal SBP < 135 mm Hg on spironolactone, compared with 42% on doxazosin, 44% on bisoprolol, and 24% on placebo.1 The effectiveness of spironolactone on SBP reduction was shown to exhibit an inverse relationship to plasma renin levels, a finding that was not apparent with the other two study drugs. However, spironolactone had a superior BP-lowering effect throughout nearly the entire renin distribution of the cohort.
The mean difference between spironolactone and placebo was –10.2 mm Hg; compared with the other drugs, spironolactone lowered SBP, on average, by 5.64 mm Hg more than bisoprolol and doxazosin; 5.3 mm Hg more than doxazosin alone; and 5.98 mm Hg more than bisoprolol alone.
Only 1% of trial participants had to discontinue spironolactone due to adverse events—the same proportion of withdrawals as that for bisoprolol and placebo and three times less than for doxazosin.1
Continue for what's new >>