The Food & Drug Administration has approved the marketing of a device that uses acoustic shock waves to boost wound closure in patients with diabetic foot ulcers (DFUs), an especially stubborn and dangerous condition.
The treatment is experimental, and only limited research into its effectiveness has been published. Still, representatives of its manufacturer say the device, known as dermaPACE, has produced promising results as a secondary treatment in stubborn cases.
A wound care specialist said in an interview that the shock wave technology appears to hold promise.
“A shortcoming in the field of wound care is that providers are typically not trained in a standardized fashion on when and how to a perform meticulous excisional sharp debridement of a wound,” said Bill Tettelbach, MD, systems medical director of Wound Care & Hyperbaric Medicine Services at Intermountain Healthcare in Salt Lake City. “In the majority of cases, the better the debridement, the more rapidly the patient will obtain wound closure.”
This new therapy may provide a benefit as a secondary treatment, especially when the patient cannot tolerate extensive sharp debridement, he said. It also could potentially improve biofilm penetration of antimicrobial topical treatments, he said.
DFUs are believed to affect as many as 1 in 4 people with diabetes over the course of their lifetimes. A 2014 report estimated that care of these wounds costs insurers as much as $13 billion a year in the U.S. alone (Diabetes Care. 2014 Mar;37[3]:651-8).
Treatment options include debridement and, in more extreme cases, hyperbaric oxygen treatment. Amputation can be required if treatment is unsuccessful.
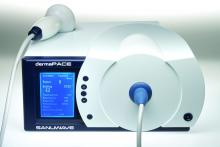
According to Mr. Stegagno, the shock wave device is about the size of a desktop computer from a decade ago. A high-voltage generator box is connected to a handheld therapy head and delivers an acoustic pulse to the patient. The system “is like a spark plug that you see in your automobile,” he said. “It’s pretty much the same technology as lithotripsy, just downsized significantly. The key part is a highly focused, high-energy pulse.”
In a news release, the FDA said it examined the results of two studies of patients with diabetes who received usual DFU care along with either the shock wave therapy or a sham therapy. A total of 336 patients took part in the multicenter, randomized, double-blind studies.
According to the FDA, the studies found a 44% wound closure rate at 24 weeks in patients who had undergone 1-7 shock wave treatments, compared with the 30% wound closure rate in those who received the sham treatment.
Side effects included pain while the device was applied, bruising and numbness, migraines, nausea, fainting, wound infection, fever, and infection beyond the wound such as cellulitis and osteomyelitis.
“There were no meaningful statistical differences in the adverse event rates between the dermaPACE-treated patients and the sham-control group,” Mr. Stegagno said. “There were no issues regarding the tolerability of the treatment, which suggests that a second course of treatment, if needed, is a clinically viable option.”
Mr. Stegagno said the FDA expressed concern about “increased incidences of osteomyelitis at later points in the trials, particularly at the 10-week mark and later.” In response to the agency’s concerns, warning statements were added to labeling, he said.
According to Mr. Stegagno, only one study into the shock wave treatment for DFU has been published, although research has been released through posters and abstracts. The small published study favorably compared shock wave therapy with hyperbaric oxygen therapy. (Diabetes Res Clin Pract. 2011 May;92[2]:187-93)
“Sanuwave will be sponsoring additional studies later this year in the [United States] as follow-on studies to the just-completed DFU trials,” Mr. Stegagno said.
The FDA says the device is intended to be used in adults aged 22 and up with certain types of chronic DFUs. The Sanuwave company says patients should be treated with 4-8 applications over 2-10 weeks.
The shock wave process appears to boost healing through a process that leads to inflammatory responses and oxygenation, Mr. Stegagno said, by first creating an initial compression phase that “squeezes the cell and creates a microtrauma.”
“The cell wakes up and says, ‘Something just punched me,’ ” he said. “This tissue and cellular disruption is believed to initiate the cellular signaling for growth factors and other proteins noted in studies.”
The effects of negative pressure also play a role in stimulation of the wound, he said.
The shock wave therapy will cost an estimated $3,000-$4,000 per protocol of 8 treatments, said Kevin A. Richardson II, the CEO and chairman of the board at Sanuwave, in an interview. The initial plan is for the company to place the devices with doctors while the firm still owns the machines, he said.
The FDA approved the marketing of the device as part of its de novo premarket review pathway, which allows certain new types of devices to be approved when approved similar devices don’t yet exist for the purposes of comparison.
Mr. Stegagno and Mr. Richardson work for Sanuwave. Dr. Tettelbach reported no relevant disclosures.