SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
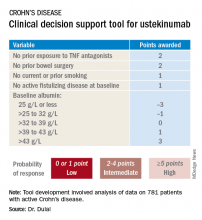
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.