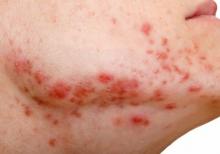
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.