Case Presentations
Case 1: Smoke Inhalation (Carbon Monoxide and Cyanide)
A 50-year-old woman was pulled from the window of a burning building and found to be in cardiac arrest with pulseless electrical activity. Standard advanced cardiac life-support was started, and infusion of intra-osseous hydroxocobalamin (OHCob) was administered at the time of intubation because of the concern for cyanide (CN) gas exposure during smoke inhalation. Return of spontaneous circulation occurred before arrival at the hospital.
Upon presentation to the ED, the patient’s vital signs were: initial blood pressure (BP), 92/47 mm Hg; heart rate (HR), 112 beats/min; respiratory rate (RR), 31 breaths/min; and temperature (T), 99.7°F. Following intubation, the patient’s oxygen saturation (SaO2) on pulse oximetry (POX) was 93%, and her fraction of inspired oxygen (FiO 2) was 100%.
On physical examination, the patient’s face was covered with soot. The lung sounds were equal and clear to auscultation bilaterally. The neurological examination was significant for a Glasgow Coma Scale of 3, without administered sedation, and there were no signs of dermal burns. Initial arterial blood gas (ABG) results were: pH, 7.06; carbon dioxide partial pressure (PCO2), 58 mm Hg; partial pressure of oxygen (PO 2), 152 mm Hg; bicarbonate (HCO 3), 17 mm Hg; SaO 2, 98% (after intubation); FiO 2, 100%; carboxyhemoglobin (COHb), 30%; and lactate, 14 mmol/L.
Case 2: Household Misadventure (Carbon Monoxide)
Several days after disabling the carbon monoxide (CO) detector in his home to silence the alarm that had continued to sound, a 67-year-old man developed weakness and called his local fire department. Upon arrival at the man’s home, the fire department confirmed an ambient air CO gas concentration over 200 ppm. Emergency medical services (EMS) promptly brought the patient to the local ED for evaluation and treatment.
Shortly after arrival at the ED, the patient’s weakness had resolved. His vital signs at examination were: BP, 154/85 mm Hg; HR, 79 beats/min; RR, 15 breaths/min; and T, 98.8°F. The patient’s COHb level was 28% with administration of 100% oxygen (O 2) via a nonrebreather mask (NRBM).
Carbon Monoxide Toxicity
Carbon monoxide is a toxin of considerable importance to emergency physicians (EPs). The diagnosis at times can be challenging, the interpretation of COHb can be confusing, and the role of hyperbaric oxygen (HBO) therapy in the treatment of CO poisoning remains controversial.
Natural Sources
Carbon monoxide is formed from the incomplete combustion of organic (carbonaceous) fuels, such as charcoal, wood, petroleum distillates (gasoline, kerosene, diesel fuel), and natural gas. Though the majority of atmospheric CO comes from natural sources (eg, volcanoes, forest fires, marsh gases), poisoning exposures are primarily due to man-made CO.
Man-Made Sources
Motor vehicle exhaust is the most abundant source of man-made CO, and exposures to exhaust fumes are common causes of both intentional and unintentional poisonings and death. Other frequent sources of CO poisoning include smoke inhalation from house fires; inadequate ventilation during use of kerosene space heaters; charcoal grills or hibachis; burning wood or charcoal; fuel-powered tools such as generators, fork lifts, and chain saws; or faulty (natural or bottled) gas appliances, such as stoves, furnaces, or water heaters ( Table 1 ). Though propane is known to burn more cleanly than natural gas (ie, less harmful to the environment), it still can produce CO.
Though neither electrical appliances nor “gas leaks” are sources of CO, like CO, natural gas (mostly methane) and bottle gas (propane) are odorless, tasteless, and colorless. Utility companies add sulfur containing mercaptans to natural gas so that leaks can be detected, but CO is only formed when the fuel is burned in a gas-powered appliance.
Endogenous Carbon Monoxide
Endogenous CO production can occur from catabolism of heme or from hepatic metabolism of methylene chloride, but exposures to this solvent are unlikely to generate COHb concentrations above 10%.
Epidemiology
The incidence of CO poisoning is likely more frequent than documented since many cases of minor exposures are unreported due to self-limiting effects and/or the vague, nonspecific nature of symptoms associated with minor exposures. In 2015, US Poison Control Centers reported over 14,000 cases of CO poisoning, only 43% of which were treated in a health care facility. 1 The vast majority of exposures (97%) were unintentional and resulted in 52 deaths (0.398%). 1
Data from hospitalized patients in 2007 revealed that over 200,000 ED visits and 22,000 hospitalizations were possibly associated with unintentional, non-fire-related CO exposures. 2 Approximately 10% of the exposures in each of these populations were confirmed by specific International Classification of Diseases Medical E codes. 2
Regardless of dataset, ED visits due to CO exposure are most common in young adults and women, occur in winter months from exposure in and around homes, and result in discharge from the ED. Elderly patients have the highest rate of hospital admission.
Carbon monoxide poisoning has long been considered a leading cause of poisoning death, though numbers appear to be declining, and CO was responsible for fewer deaths than opioids in 2017.2 The National Center for Health Statistics reported 56,133 CO-related deaths from 1979 through 1988—an average of 5,600 per year. 3,4 Of these, 46% were from suicide; 28% were related to burns or house fires; and 21% (11,547) were characterized as unintentional. Motor vehicle exhaust was associated with 57% of the unintentional deaths. A more recent analysis of unintentional exposures reported 2,244 deaths during the period of 2010 to 2015—an average of 374 deaths per year (393 in 2015). 5
Preventive measures are likely responsible for the significant decline in non-fire-related CO poisoning deaths from the early 1970s through the 1990s. The introduction of catalytic converters in automobiles in 1975 and O 2 sensors in 1981 eventually reduced automotive CO emissions by 95% compared to pre-1975 vehicles. 6 Both unintentional death and suicide rates associated with CO from motor vehicles subsequently declined by 81% and 43%, respectively. The lower decline in suicidal deaths serves as a reminder that intentional exposure to motor vehicles remains dangerous and potentially lethal.
Pathophysiology/Mechanisms of Toxicity
Carbon monoxide is a colorless, odorless gas that readily reaches the bloodstream during alveolar gas exchange. Since absorption is rapid, exposures to high CO concentrations can produce toxicity within minutes, though exposure severity is related to both inspired CO concentration and duration of exposure.
Endogenous Elimination
Carbon monoxide is eliminated from the body in expired air, with an elimination half-life dependent on FiO 2 and atmospheric pressure. Accordingly, COHb decreases with a half-life (all approximate) of 4 to 6 hours when patients are breathing room air (21% O 2), 60 to 90 minutes with O 2 delivery at 95% to 100%, and 20 to 40 minutes under hyperbaric conditions (2.5-3.0 atmospheres absolute [ATA]).
Effect on Hemoglobin
Once absorbed, CO has an affinity for hemoglobin (Hb) that is over 200 times greater than does O 2.7 The formation of COHb results in both a decreased O 2-carrying capacity of Hb at the sites where O 2 would have been, and because of its new configuration, COHb does not allow currently bound O 2 to be offloaded. This is graphically represented by a shift of the O 2-Hb dissociation curve to the left. In addition, CO continues to be bound by other intracellular heme molecules in myoglobin of skeletal and myocardial muscle, and the cytochrome oxidase system in mitochondria. 8
Immunologic and Inflammatory Effects
Carbon monoxide poisoning results in a cascade of immunologic and inflammatory effects, such as generation of nitric oxide, lipid peroxidation from neutrophils, mitochondrial oxidative stress, and apoptosis. These effects result in cellular asphyxia in all organs, but the most emergent life-threatening concerns are ischemia to the brain and heart.
Severity of Toxicity and Exposure
As previously noted, the severity of CO poisoning is dose-dependent, meaning that it is related to the concentration of CO in inspired air and the duration of the exposure. Carbon monoxide is typically absent in fresh air, but levels may approach 2 to 5 ppm due to cooking, wood burning, mild air pollution, etc. The source of levels above 5 ppm should generally be investigated.
Maximum safe exposure levels for workers over an 8-hour period range from 25 to 50 ppm. Exposures to CO levels above 50 to 100 ppm are likely to elicit symptoms in most patients, depending on duration of the exposure. Carbon monoxide levels of 200 ppm may result in a mild headache after 2 to 3 hours of exposure, and a more severe headache and nausea after 1 to 2 hours of exposures to 400 ppm of CO.
Accordingly, home CO detectors use a combination of ppm and time for alarms, and they may not sound an alarm at 40 ppm until the level persists for 8 or so hours. Home CO detectors, however, will sound an alarm immediately when a level of 80 to 100 ppm is reached.
Clinical Presentation
Acute Exposure
Acute exposure to CO causes a variety of effects that are largely nonspecific, as there is no toxic syndrome (toxidrome) considered pathognomonic for CO poisoning. Ambient CO levels, duration of exposure, minute ventilation, presence of other toxic gases, and patient comorbidities can all contribute to the severity of exposure and presenting signs and symptoms. Effects associated with mild poisoning include headache, dizziness, blurred vision, fatigue or weakness, nausea, and shortness of breath. Patients with pre-existing respiratory, cardiovascular (CV), or neurological compromise are likely to present with more pronounced symptoms. In either case, these complaints may easily be confused with a viral illness, emphasizing the importance of eliciting a history of potential exposure to CO, particularly when multiple patients are involved.
As the concentration of COHb increases, more significant clinical effects can be expected, including tachycardia, chest pain, hypotension, dysrhythmias, lethargy, coma, apnea, and seizures. Hypoxia can result in myocardial injury, cerebral edema, stroke, and acute pulmonary and kidney injury.
Following acute exposure, the severity of effects correlates with the peak pretreatment COHb concentration. However, the peak concentration is usually unknown, since most patients with significant exposures will have some time period elapsed between the exposure and the determination of COHb, and the COHb will have declined at a rate depending on FiO 2 and minute ventilation. In these circumstances, COHb is a poor indicator for HBO therapy and outcome.
Delayed Neurological Sequelae
Persistent, recurrent, or delayed (following period of no symptoms) neurological effects can occur in up to 40% of cases, and patients with significant exposures (eg, loss of consciousness) appear to be at greatest risk. These effects most often occur within the first 3 weeks following exposure, and have been known to persist for months to years. Such effects include headache, dizziness, impaired memory or cognition, and emotional lability. Predicting which factors in CO exposure and/or treatments can be modified to prevent neurological sequelae remains challenging.
Diagnostic Testing
Pulse Co-oximetry
Prehospital care POX typically reads COHb as oxyhemoglobin, thereby displaying a normal SaO 2.9 Noninvasive CO pulse co-oximetry using a pulse oximeter (Rad-57, Masimo Corporation) provides a reading between –6 to +4 of the true COHb with a false-positive rate of 11% and false-negative rate of 46%. 10 This high false-negative rate makes noninvasive CO pulse co-oximetry a poor tool to rule out a CO exposure. 11 If OHCob has been administered due to concerns for CN poisoning (smoke inhalation), concentrations of COHb detected by a co-oximeter medical device may be decreased, as noted by a mean decrease of 1% in healthy volunteers exposed to OHCob only. 12
Venous and Arterial Blood Gas Testing
For a patient in the hospital, exposure to CO can rapidly be determined using co-oximetry to measure COHb in a venous or arterial sample. Obtaining a venous sample may be a more practical approach, as other venous measurements will likely also be obtained. Baseline “normal” COHb levels should be less than 5%, but may be up to 8% to 10% in tobacco smokers.
Other Laboratory Studies
Other important laboratory tests that should be obtained are a complete blood count, lactate level, venous blood gas, and basic metabolic panel (to assess acid/base status). In two retrospective studies of patients exposed to CO, elevated lactate levels were associated with altered mental status. 13,14 However, elevated lactate levels were not seen in a majority of patients with CO poisoning.
In addition, CN exposure should be considered when the lactate level is greater than 8 mmol/L, particularly in patients with smoke inhalation. 15 Troponin I and creatinine phosphokinase tests can be used to screen for myocardial or skeletal muscle injury.
Effect of Hydroxocobalamin on Laboratory Evaluation
It is important to be cautious when interpreting the results of laboratory studies in patients who have been given OHCob due to the potential co-exposure to CN (smoke inhalation). The red discoloration of body fluids after OHCob administration makes laboratory evaluation by spectrophotometric techniques erroneous. 16 Of greatest concern is the accuracy of COHb concentrations. 17 In a study using rabbit models by Lee et al, 17 OHCob administration was shown to falsely increase COHb concentrations.
Livshits et al 18 reported conflicting effects on COHb in two human cases. In the first case, the patient’s true COHb was 93% lower (2.5% vs 34.9%) following administration of 5 g of OHCob, as measured with a rapid blood gas analyzer. In the second patient, COHb was 76% lower (10.7% vs 44%) following OHCob administration, which was also measured by a blood gas analyzer. Both of these cases illustrate lower true COHb concentrations than would be expected following the administration of only supplemental O 2.
In a controlled experiment by Pace et al 19 examining the effects of OHCob on measurement of COHb at both physiological (3%) and pathological (30% and 50%) concentrations in human blood samples, the degree of interference depended on the type of co-oximeter used, the degree of COHb elevation (at pathological levels only), and the concentration of OHCob added. Other studies, including an evaluation of OHCob interference by Carlsson et al 20 using nine different analyzers have confirmed the interference of OHCob on photometric assays. Of particular clinical importance, a falsely increased lactate level was seen after true lactate levels were found to be below 4.8 mmol/L (but not greater) using spectrophotometric or electrochemical detection. 21 This increase in the false-positive assessment of the degree of toxicity could lead unnecessary escalation of care.
These studies emphasize the need to exercise caution when interpreting laboratory test results following OHCob administration. Ideally, it would be best if blood samples were obtained prior to OHCob administration by EMS or in the ED, if the clinical scenario allows it.
Imaging Studies
For patients presenting after a closed-space fire, a chest radiograph will help assess for pulmonary injury. The classic finding of CO poisoning on head computed tomography (CT) and magnetic resonance imaging scans is evidence of ischemia in the basal ganglia. The radiographic findings may help determine the diagnosis of the altered mental status patient who presents without a history. An electrocardiogram (ECG) is also useful for detection of myocardial ischemia or dysrhythmias, when signs or symptoms of either are possible from history and physical or cardiac monitoring.
Intentional Inhalation
Intentional inhalation of fumes containing CO is a relatively common mechanism for suicide. In patients who survive, it is important for EPs and other providers to suspect additional means of self-harm. For example, at our institution, we have encountered several patients with self-inflicted trauma after remaining conscious following a medication overdose. Accordingly, patients who have intentionally inhaled CO should also be evaluated for occult medication poisoning (and trauma).
Treatment
The first step in treating a patient with CO poisoning occurs prior to arrival at the ED, when he or she is removed from continued exposure. The second step is assessing whether this is only a CO exposure, or a mixture of gases from combustion in a closed space, that might also contain CN. When CO and CN are combined OHCob is indicated to treat CN toxicity.
Additionally, if the patient is brought to the ED via EMS, O 2 therapy will most likely have been initiated en route. In either case, the concentration of COHb may not accurately reflect the magnitude of exposure or prognosis, and should not be used to dictate the level of therapy or disposition. The patient’s vital signs and clinical findings of end organ toxicity should guide the appropriate supportive care.
Supplemental Oxygen Therapy
Initial administration of 100% O 2 during assessment of airway, breathing, and circulation is the first step in accelerating the removal of CO from Hb. For patients suffering from smoke inhalation, assessment and establishment of a secure airway when there are signs of soot or burns in the airway must always take precedence over other actions. Continuous cardiac monitoring, POX, observation, and establishment of intravenous access are often needed for detection and management of CV instability or change in mental status in cases of moderate-to-severe CO exposures. Mild exposures with headache, nausea, and flu-like symptoms can be managed with symptomatic treatment and normobaric O2 until resolution of symptoms and improvement in COHb occur.
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy involves the delivery of high-flow O 2 (typically at 100%) under increased atmospheric pressure (2.5 -3.0 ATA). Oxygen delivered at ambient air pressure (1.0 ATA) is often referred to as normobaric oxygen. Although HBO is best known for its ability to enhance CO elimination, research points to a much more eloquent mitigation of CO toxicity on the molecular level. These mechanisms include an increased amount of dissolved O 2 in blood, regeneration of cytochrome oxidase, decreased leukocyte adhesion to microvascular endothelium in the brain, decreased lipid peroxidation in the brain after loss of consciousness, and preservation of adenosine triphosphate. 22
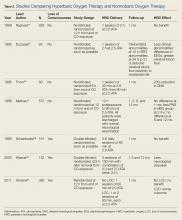
For most patients, the majority—if not all—of COHb will be eliminated by the time they present to a suitable HBO chamber. Despite the knowledge that HBO therapy has a positive toxicokinetic effect by increasing the elimination of CO, all of the major, prospective studies on the usefulness of HBO are related to prevention of neuropsychiatric sequelae mediated by immunological and inflammatory effects. The role of HBO in the treatment of CO poisoning has been debated for decades. Multiple studies that differ in methodology, patient populations, delivery of HBO treatments, and assessment of benefits fail to provide a consensus on the role of HBO therapy ( Table 2 ).23-30
Before transferring a patient to a facility for HBO therapy, the potential risks and benefits of transport must be considered. In a 10-year retrospective study by Sloan et al 31 of 297 CO-poisoned patients (mean COHb, 38%) 46% of patients had cardiopulmonary and neurological complications prior to HBO therapy at some point in the transfer pathway. During HBO therapy, 18% of patients had complications that included emesis, agitation requiring sedation, seizures, hypotension, tension pneumothorax, cardiac arrest, cardiac arrhythmias, and myocardial ischemia. It is therefore incumbent that personnel attending patients undergoing HBO therapy for CO poisoning be aware of, and able to manage, this variety of serious effects.
When an HBO chamber is at a clinical site with experts in the field and staff available 24 hours a day, the decision to utilize HBO may easily be made without obstacles. For most EPs, however, this is not the case. Locating and transferring a patient to an HBO center is typically a considerable logistical challenge. For many rural facilities, HBO is just not a timely therapeutic option. Two studies state the benefit of HBO therapy is greatest when starting within 6 hours from the end of the CO exposure. 24,26
Identifying those CO-poisoned patients who meet evidence-based criteria for HBO is difficult. Patients with mild CO poisoning will do well without HBO, and critically ill patients will probably not consistently benefit from HBO. However, a pragmatic solution must be considered when efficacy studies are incongruent with conflicting results. When signs of end-organ toxicity from CO are present, but cardiac arrest has not yet occurred and the logistics are streamlined, the benefit of HBO may outweigh the risk.
Signs of end-organ toxicity include syncope, seizures, coma, ischemic changes on ECG, and pregnancy with unresolved maternal distress or fetal distress. Although a COHb level greater than 25% or 15% (pregnant) alone is commonly used as an indication for HBO, this is largely based on opinion. Conversely, HBO is unlikely to be helpful in patients who have been resuscitated after CO-related cardiac arrest. 32
Treatment Guidelines
The American College of Emergency Physicians recently developed a position statement regarding the management and treatment of CO poisoning. 33 The clinical policy addresses several of the controversies discussed in this review, and provides a level of evidence for each response ( Table 3 ).
Case Conclusions
Case 1 (Smoke Inhalation Due to CO and Cyanide Poisoning)
The patient in this case suffered severe CO and CN toxicity. A head CT scan revealed diffuse edema consistent with anoxic brain injury. After conferring with the family regarding the patient’s condition and prognosis, the decision was made to withdraw life-sustaining therapy and support, and the patient died.
Case 2 (Household Misadventure)
The patient in this case was successfully treated with 100% O 2 via a NRBM and was subsequently discharged home within 4 hours from presentation.
Conclusion
Exposures to CO are ubiquitous due to our heavy reliance on carbon combustion, and the manifestations of CO toxicity are protean. Therefore, CO poisoning must be considered more frequently in the differential diagnosis of indiscriminant symptoms affecting the neurological, cardiac, pulmonary, and gastrointestinal systems, especially when multiple patients have similar symptoms.
The diagnosis of CO poisoning is straightforward when a serum COHb level is obtained on a venous or arterial blood sample. Treatment starts when the patient is removed from further CO exposure and breaths normobaric oxygen at ambient levels or supplemented. Because there is no clear evidenced-based indication for HBO therapy, further treatment with HBO is naturally limited by rational constraints.