NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
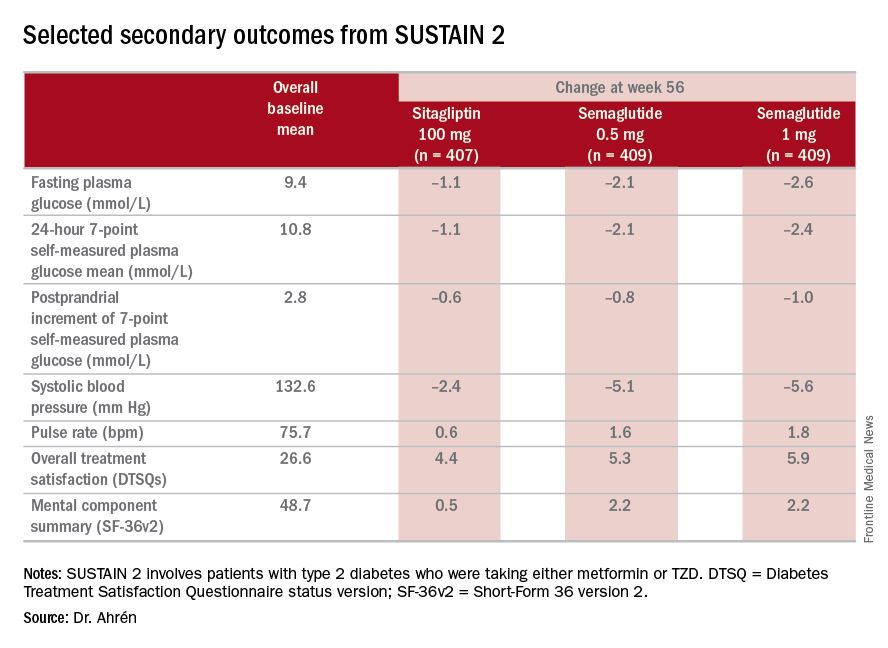
Bo Ahrén, MD, of Lund University in Sweden presented results from SUSTAIN 2, a double-blind substudy of SUSTAIN that includes 1,231 patients with type 2 diabetes taking either metformin or TZD (1,225 were exposed to treatment). The patients were randomized to three nearly equal arms of daily sitagliptin 100 mg, or weekly semaglutide 0.5 mg or 1 mg.“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.
Dr. Ahrén noted that 69% of the 0.5-mg semaglutide group and 78% of the 1-mg group achieved HbA1c below the 7% target while 36% of those on sitagliptin did. The semaglutide groups also showed superior weight loss: an average of 4.3 kg and 6.1 kg for the 0.5-mg and 1-mg groups, respectively, vs. 1.9 kg with sitagliptin. “On the 1-mg dose of semaglutide, one-fourth of the patients had reduction of more than 10% of their weight, compared to 3% with sitagliptin,” he said.The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.