In an era of potent antiretroviral therapy (ART), end stage liver disease (ESLD) is the second leading cause of death among patients with human immumodeficiency virus (HIV) infections who are co-infected with hepatitis C virus (HCV).1,2 In the U.S., HCV infection is the major cause of liver failure, liver transplantation, and liver disease associated death, with an annual mortality that now exceeds that of HIV.1,2 It is estimated that 30% to 35% of persons with HIV infection also have HCV infection.1,2 Interestingly, patients with underlying HIV have a lower rate of spontaneous hepatitis C virologic clearance during acute infection, which is thought to be directly related to their level of underlying immune suppression and immune function.3 The consequences of HCV infection in this population are significant and include accelerated liver disease and fibrosis progression, higher rates of ESLD, and shortened life span after hepatic decompensation.4,5 Because of these complications, the U.S. Department of Health and Human Services (DHHS) recommends that all HIV-infected patients should be screened for HCV infection, preferably before starting ART.6
HCV Treatment for HIV-HCV Co-Infection
The higher rates of fibrosis progression, decompensated liver disease, and morbidity and mortality secondary to ESLD compared with patients with HCV monoinfection are a major impetus to treat HCV in patients with HIV-HCV co-infection. However, there are very limited data on the safety and efficacy of the available antiviral agents for co-infection. The current paradigm is to stage patients before HCV treatment since those patients with minimal fibrosis may be able to wait for future less toxic or complex therapies, while HCV therapy should be offered to patients with portal or higher stages of fibrosis.7 Today with simpler and more effective therapies, even this paradigm is rapidly shifting to offering treatment for all infected patients.
The goal of HCV therapy is to achieve sustained virologic response (SVR), undetectable HCV RNA levels 24 weeks after the end of treatment. This endpoint is the gold standard of treatment-induced viral eradication and is used as the primary endpoint for treatment of HCV regardless of HIV status. Secondary goals are achieved upon viral eradication, including the reduction in morbidity and mortality associated with liver disease (eg, fibrosis/inflammation, hepatocellular carcinoma [HCC]).8 Failure to achieve SVR leaves the patient with continued risk of liver disease progression, including fibrosis and liver decompensation. Interestingly, in patients who have received treatment with peginterferon and ribavirin, there are data supporting a lower risk of liver-related mortality, hepatic decompensation, and liver stiffness when initial SVR is achieved, but there is a subsequent relapse (reappearance of HCV RNA).9
The complex decision to treat a patient with HIV infection for HCV is based on the stage of liver disease along with HCV genotype, viral load (VL), presence of comorbidities, and stability of the patient’s HIV regimen (if any). This is a long-term commitment for the patient, and his or her readiness to undergo and adhere to therapy is as important as the regimen. Given this complexity, it is also clear that treatment of HCV in either HCV alone or HIV-HCV co-infection should not be attempted without consultation and guidance from an infectious diseases or gastroenterology physician who has experience and training in the management of HCV and HIV. In general, a team of providers, including the physician, pharmacist, and social worker are required to ensure safety, compliance, and efficacy.
The timing of treatment is important in patients with bridging fibrosis or cirrhosis. However, treatment of mild to moderate disease can also be justified given the data supporting more rapid disease progression in patients with HIV-HCV co-infection, although not as pressing.4,5 The best indicator for disease stage remains liver biopsy, which is assessed for grade and stage of the liver injury. There are 3 primary reasons for performing a liver biopsy: (1) It provides useful information on the status of the liver injury; (2) It identifies features useful in the decision to initiate therapy; and (3) It may reveal advanced fibrosis or cirrhosis that necessitates surveillance for HCC. The biopsy also provides information on other histologic features that might have a bearing on liver disease progression.10 However, there are clear contraindications to treatment, including a history of decompensated cirrhosis (may be exacerbated by treatment), pregnancy (teratogenicity of ribavirin), or uncontrolled depression and unstable cardiac or pulmonary conditions.6 Patients with untreated/uncontrolled HIV and/or AIDS are not optimal for treatment.10
In patients presenting with HIV and HCV coinfection the order of HIV or HCV treatment does affect patient outcomes.6 Recommendations from the DHHS HIV task force are that all treatment-naive HIV/HCV coinfected patients be initiated on HIV ART regardless of their CD4 cell count.6 In patients with lower CD4 counts (eg, < 200 cells/mm3), it may be preferable to initiate ART and delay HCV therapy until CD4 count increases. The importance of immune reconstitution and function has been demonstrated by studies that show ART may slow the progression of liver disease by preserving or restoring immune function and reducing HIV-related immune activation and inflammation.
There is also evidence to support the model that HCV viremia increase proportionally to the extent of CD4 cell count decline; although there is no direct correlation between extent of liver damage and viremia there are direct implications for therapy.10 For most HIV-HCV co-infected patients, including those with cirrhosis, the benefits of ART outweigh concerns regarding drug-induced liver injury. Therefore, ART should be considered for patients with HIV-HCV co-infection, regardless of CD4 count, and treatment of HCV may be deferred until there is a rise in the CD4 count.11,12
Current Treatment Regimens
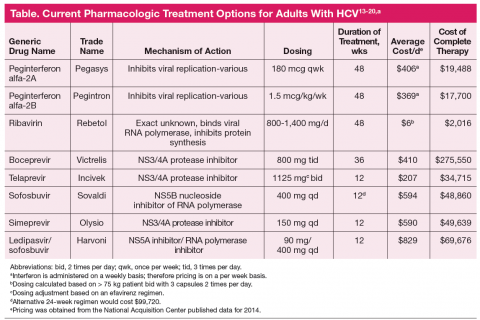
Antiretroviral regimens for patients with HIV-HCV coinfection are generally the same as those for individuals without HIV infection (Table).13-20 Importantly, when treating both infections the provider must consider the large pill burden, drug-drug interactions, and overlapping toxicities (in particular hepatic) prior to
starting therapy. The same predictors as those for monoinfection apply to co-infected patients, including the level of baseline viremia, with high-level viremia having a negative impact on overall SVR rates in clinical trials with the new protease inhibitor boceprevir (although it did not impact telaprevircontaining
regimens).21,22 Another factor that predicts treatment response is the HCV genotype (total of 6). In the U.S., genotype 1 accounts for 70% to 75% of HCV infections. Genotype 1a is responsible for two-thirds, and genotype 1b for one-third of genotype 1 infections. In treatment studies to date, genotype 1b is less likely to develop viral drug resistance and therefore has a higher treatment cure rate than HCV genotype 1a.
Treatment success with interferon-based regimens is also influenced by the patient’s interleukin 28B gene (IL28B) polymorphism. Patients with the IL28B CC genotype have a greater response than those with IL28B TT genotype.23 IL28B is a member of the type III interferon system. Interferons are part of the innate immune response of host cells to pathogens in particular viruses. They establish an antiviral state by inhibiting protein synthesis, alerting the immune system to the presence of pathogen, and inducing proteins with antiviral activity. Interferons are pivotal factors in fighting viral infections and establishing an antiviral state
in the infected and surrounding cells.24 Currently, we do not routinely determine IL28B genotypes; however, as technology improves and genotyping becomes more commercially available, this may be incorporated into routine practice. Moreover, with the second generation HCV drugs, including simpeprevir and sofosbuvir, the IL28 genotype is not a factor as new recommendations include interferon-free regimens.25
Peginterferon alpha plus ribavirin has been the mainstay of therapy since 2001, producing an overall 40% response in treated patients with HCV genotype 1.26 More recently, 4 classes of direct-acting antiviral (DAA) agents have been developed, including nonstructural protein 3/4A (NS3/4A) protease inhibitors (PIs), nonstructural protein 5B (NS5B) nucleoside inhibitors, NS5B non-nucleoside inhibitors, and nonstructural protein 5A (NS5A) inhibitors. Of these classes, the PIs boceprevir and telaprevir have been in use since 2011 and have significantly improved response rates to therapy for genotype 1 infection. These drugs, however, continue to require peginterferon and ribavirin therapy; therefore many of the barriers to therapy that existed previously remain.
Several clinical trials demonstrated significantly increased efficacy with PI backed therapy when compared with standard interferon ribavirin regimens in treatment-naive and treatment-experienced nonresponders/patients who have relapsed.21,22,27 Previously one of the main treatment predictors was the rapidity of viral suppression assessed at 4 weeks of therapy. If patients have a rapid virologic response (ie, > 2 log decrease in VL), they generally do well with sustained viral suppression. However, this predictor becomes unclear as the DAA are used. Because of their potency with rapid viral suppression, the durability of the rapid virologic response and its use as a predictor of treatment success becomes uncertain. To date, there have been few clinical trials completed and published on the efficacy and safety of this triple drug regimen in the HIV-HCV co-infected patient. However, what data there is available is encouraging.
In a phase 2 clinical trial of genotype 1 co-infected patients after a 4-week peginterferon and ribavirin leadin, boceprevir was initiated and continued for 44 weeks vs continuation of standard of care with peginterferon and ribavirin. In this cohort 42% of patients in the boceprevir arm achieved viral suppression at week 8 vs 15% in the standard therapy arm. At 24 weeks postcompletion of therapy, 63% of patient maintained SVR compared with 29% in the standard arm. Importantly, co-infected patients did not have new previously unreported adverse events (AEs), and the percentage of patients with AEs seemed to be similar to those with HCV infection alone.28 Similar studies of telaprevir also demonstrated more effective therapy than did the standard of care in co-infected patients. Another study evaluating telaprevir utilized a small cohort of 60 patients with genotype 1 infection. They were treated for 12 weeks with peginterferon, ribavirin, and telaprevir followed by an additional 36 weeks of peginterferon and ribavirin. After 4 weeks of therapy, 68% of patients in the telaprevir arm had rapid virologic response compared with none of the patients in the standard therapy arm. Patients included in this cohort had higher VL and advanced liver disease. Upon completion of the study at both 12 and 24 weeks posttherapy, 74% vs 24% of patients had SVR, respectively. Safety data in this study also demonstrated similar toxicity profiles as those with HCV monoinfection.29
In recent months the FDA approved the protease inhibitor simeprevir with pegylated interferon and ribavirin for the treatment of patients with HCV genotype 1. In addition, the FDA also approved sofosbuvir with pegylated interferon and ribavirin for the treatment of HCV genotype 1, and sofosbuvir and ribavirin for the treatment of HCV genotypes 2 and 3. Oral sofosbuvir plus ribavirin in patients with HCV and HIV infection (CD4 count > 500 cells/μL) resulted in an SVR of 76% in patients with genotype 1, 88% in those with genotype 2, and 67% in those with genotype 3.30 The sofosbuvir regimen utilized in the PHOTON-1 trial is an interferon-free regimen, the first time there has been success with interferon-free regimens in this population.30 A new study released in early November at the American Association for the Study of Liver Diseases meeting presented data from the ERADICATE trial, which was designed to test a simple once daily regimen of sofosbuvir and ledipasvir (a new inhibitor of HCV NS5A, a viral phosphoprotein that plays an important role in viral replication, assembly, and secretion) in 50 patients. Results presented demonstrated that at 12 weeks posttreatment all patients in the ART untreated group and all but one in the ART treated group had undetectable HCV RNA. The overall SVR rate was 98% for this study. This is the first time an interferon and ribavirin-free regimen has shown clear efficacy in the treatment and cure of HCV infection.25 New guidelines for the treatment of patients with HCV-HIV co-infection are expected to be released in the immediate future.
Conclusion
The sudden and dramatic change in hepatitis C management is now here, with an extraordinary array of new drugs expected to cure the majority of hepatitis C-infected patients.31 It remains an exciting time in the field as for the first time we may be able to reach solid cure rates with simpler, more accessible regimens, in particular as we are on the threshold of totally oral therapy. One of the greatest limitations has been and will remain the economics of therapy (Table), which is not discussed here but remains at the forefront of barriers to care and cure.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.