Data Sources and Chart Abstraction
Data sources included the VA Corporate Data Warehouse (CDW), the VAPSHCS cancer registry, the Computerized Patient Record System (CPRS), VistA (Veterans Health Information Systems and Technology Architecture), VistA Web, and VistA Imaging.
Study Population
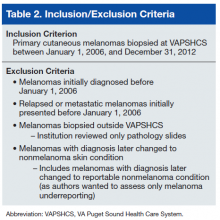
The study population consisted of veterans who had been diagnosed with primary cutaneous melanoma and had the diagnosis confirmed by biopsy performed at VAPSHCS between January 1, 2006 and December 31, 2012.
Statistical Analysis
Odds ratios were calculated using the MedCalc Odds Ratio Calculator. 14
Methods
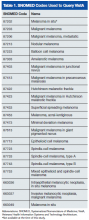
The authors identified SNOMED (Systematized Nomenclature of Medicine) codes that included the character string melanoma (Table 1). Using these codes, they queried VistA to identify melanoma cases diagnosed in the VAPSHCS Pathology and Laboratory Service during the period 2006 to 2012. To confirm the completeness of the local report, the authors performed the same search using the CDW. The SNOMED code case-finding was supplemented with cases ascertained using ICD-9 codes and problem list diagnoses.
A case must be reported to the local cancer registry if diagnosis or treatment takes place at the facility. All cases ascertained with the authors’ search criteria are, by definition, reportable to the local cancer registry. The authors then applied inclusion and exclusion criteria to determine
which cases were primary cutaneous melanomas and therefore candidates for this investigation (Table 2).
Having ascertained the primary cutaneous melanomas, the authors abstracted the pathology TNM (tumor, node, metastasis) staging (Breslow depth, mitotic index, presence of ulceration) and diagnosis dates from CPRS pathology reports. They then determined whether each case had been reported to the VACCR (OncoTraX was used to query for the accession status of each melanoma). If the melanoma was not accessioned, the authors tried to determine why.
Results
The authors discovered 193 primary cutaneous melanomas diagnosed by biopsy performed at VAPSHCS. Of these 193 melanomas, 71 (36.8%) had not been reported.
After the pathologist has completed a report, SNOMED codes are assigned by the pathology laboratory. Case finding with OncoTraX depends on SNOMED codes and other parameters (imaging, treatment, oncology consultation). OncoTraX is designed for case finding using World Health Organization (WHO) standardized 8000/X-9000/X series SNOMED codes. To understand the relationship between reporting and SNOMED codes, the authors ascertained the codes for all melanomas in the present study.
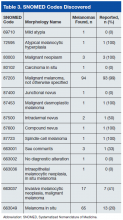
Table 3 lists the SNOMED codes assigned to melanomas biopsied at VAPSHCS and the percentage reported for each. Of the 106 melanomas that had been assigned WHO standardized codes, 101 (95.2%) had been reported. In contrast, only 21 (24.1%) of the 87 melanomas that had been assigned non-WHO standardized codes had been reported. In this study, non-WHO standardized codes are locally generated codes; they began with facility station number 663.
Use of locally generated codes may have contributed to nonreporting. Of the 71 melanomas not reported, 66 had a SNOMED code beginning with 663, and the other 5 had a WHO standardized SNOMED code. Odds of being nonreported were much higher for the melanomas with 663 codes than for the melanomas with WHO standardized codes (odds ratio [OR], 63.5; confidence interval [CI], 22.8-176.7; P ≤ .0001).