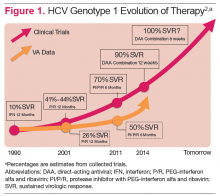
Hepatitis C virus (HCV) infection remains a significant problem in the VA system, with over 174,000 current actively infected patients. 1 Despite the availability of antiviral treatment since the early 1990’s, only approximately 26% of patients have ever been treated. These treatments required the use of pegylated interferon alfa (PEG) and ribavirin (RBV), which are associated with significant adverse events (AEs) that prevented many from receiving the treatment. Of those treated, only a minority achieved a sustained virologic response (SVR), due to the limited efficacy of the treatments (Figure 1). 2 With the advent of new direct-acting antiviral (DAA) treatments in 2011, treatment efficacy improved.
The first DAAs in use were the viral nonstructural protein 3/4A (NS3/4A) serine protease inhibitors (PIs) boceprevir and telaprevir, which were used with PEG and RBV for patients with HCV genotype 1 infection. This combination therapy improved SVR rates from about 26% to 50% in patients with HCV genotype 1 in the VA. 3,4 However, due to the significant AEs with these combinations, relatively few patients were treated.
In late 2013, the FDA approved other DAAs, which allowed patients to be treated effectively without PEG. These included the nucleotide nonstructural protein 5B (NS5B) polymerase inhibitor sofosbuvir and a secondgeneration NS3/4A PI simeprevir. 5-7 The first nucleotide analog NS5B polymerase inhibitor, sofosbuvir and the nonstructural protein 5A (NS5A) replication complex inhibitor, ledipasvir, was approved in October 2014. 8-10 The recent developments in noninterferon treatments have been accompanied by revised treatment guidelines or recommendations by major professional societies. Current treatment recommendations will be reviewed here, but the recommendations will continue to evolve as new DAAs come to market.
DAA Sites of Action
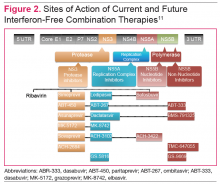
The HCV genome is a positive-stranded RNA molecule of about 9,500 nucleotides, which encodes a polyprotein of approximately 3,000 amino acids that form 10 individual viral proteins. These are composed of both structural and nonstructural (NS) proteins that are responsible for replication of the genome and formation of new viral particles. Understanding of the HCV-encoded proteins and their functions has permitted the development of different DAA therapies. In general, targeting a single protein is not effective, and combination therapy targeting 2 proteins is required for viral eradication (Figure 2). 11 The 3 drug targets that are currently available include NS3/4A serine PIs (eg, simeprevir, boceprevir, telaprevir), NS5A replication complex inhibitors (eg, ledipasvir, daclatasvir), and NS5B RNA-dependent RNA polymerase inhibitors (eg, sofosbuvir).
Other DAA’s are in development that have targets in host rather than viral cells. These include cycolphilin A inhibitors and the micro-RNA (miR-122) antagonist miravirsen. 12,13
The RNA-dependent RNA polymerase, encoded by the HCV NS5B is targeted by 2 classes of inhibitors: nucleoside or nucleotide analog inhibitors (NIs), and non-nucleoside inhibitors (NNIs). 11 The only NI of the NS5B protein approved by the FDA is sofosbuvir. The resistance profiles of NIs and NNIs differ, because they bind to distinct sites on the NS5B protein. NIs are analogs of natural substrates and bind to the active site of the RNA polymerase, whereas NNIs are allosteric site inhibitors. NIs have activity in vitro against all HCV genotypes and have high barrier to resistance as the active site of NS5B polymerase is less tolerant of different amino acid substitutions.