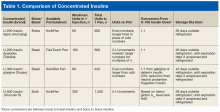
For a long time, 500 U/mL (U-500) insulin was the only concentrated insulin available on the market. With many diabetes mellitus (DM) patients requiring larger doses, additional 200 U/mL (U-200) and 300 U/mL (U-300) concentrations became available. As clinical guidelines lack specific recommendations for optimal use of U-200 and U-300 insulins, clinical discretion is warranted in identifying patients for whom use of these insulins is appropriate. U-500 insulin is recommended in cases that require ≥ 200 U/d or > 2 U/kg/d. Given the ongoing DM and obesity epidemics, increased use of concentrated insulins is likely. Clinicians must stay well informed about the characteristics and benefits of concentrated insulins to remain confident recommending, prescribing, and adjusting these medications.
U-200 Insulin Lispro
Pharmacokinetics/Pharmacodynamics
The amino acid structure of U-200 insulin lispro is different from that of endogenous insulin. In U-200 lispro, lysine replaces a proline at position B28, and proline replaces a lysine at position B29.
U-200 lispro is a bolus insulin with pharmacokinetics (PK) and pharmacodynamics (PD) similar to those of U-100 lispro: onset of action, ~15 minutes; time to peak, 30 to 90 minutes; and duration of action, 4 to 5 hours. U-200 lispro should be administered either 15 minutes before a meal or immediately after a meal.1
In a euglycemic clamp study of patients without DM, a 20-U dose of U-200 lispro and a single 20-U dose of U-100 lispro were found to have similar mean area under the glucose infusion rate curves, mean area under the serum insulin concentration-time curves from time 0 to infinity, mean peak serum insulin levels, and time to maximum glucose-lowering effects.1 For both U-200 lispro and U-100 lispro, time to maximum effect was 1 hour.2
Even numbers are marked on the dial of the pen. Odd numbers are not marked, but longer lines appear in their place. U-200 lispro should not be mixed with any other insulin, whereas U-100 lispro can be mixed with neutral protamine Hagedorn insulin.
Safety/Efficacy
There has been 1 bioequivalence study of euglycemic patients without type 2 DM (T2DM) but no studies of the safety or efficacy of U-200 lispro in patients with DM.3,4 U-100 lispro converts 1:1 to U-200 lispro (eg, 60 U of U-100 lispro converts to 60 U of U-200 lispro).1 The volume of U-200 lispro would be smaller than that of U-100 lispro.
Economic Analysis
There are no published U-200 lispro economic analyses.
Dosing
U-200 lispro should be converted from other bolus insulins in a 1:1 ratio.1
Recommendations
Definitive recommendations await efficacy trials comparing use of U-200 lispro and other bolus insulins in patients with DM. Currently, U-200 lispro may be considered for patients with DM who require high doses of bolus insulin and who may benefit from smaller volumes of lispro.
U-200 Insulin Degludec
Pharmacokinetics/Pharmacodynamics
The basal insulin degludec (Tresiba) is available in U-100 and U-200 concentrations in a pen. After subcutaneous injection, degludec forms gradually dissociating multihexamer chains, which account for its flat and stable PK/PD profile. U-100 degludec and U-200 degludec have similar duration of action (≥ 42 hours) and time to steady state (2-3 days).5,6 A patient who misses a regularly scheduled dose should allow at least 8 hours between injections. Taking degludec at variable times does not decrease efficacy as long as this 8-hour minimum interval is observed.7
Safety/Efficacy
During its development, degludec was evaluated in more than 5,000 patients across 11 therapeutic trials.8 The key studies that led to the approval of degludec used insulin glargine as a comparator. In a 52-week study of 1,030 insulin-naïve patients with T2DM, degludec was noninferior to glargine in hemoglobin A1c (HbA1c) reduction (1.06% vs 1.19%). Overall hypoglycemia rates were similar, though there were fewer nocturnal hypoglycemia episodes with degludec than with glargine (0.25 vs 0.39 per patient-year of exposure; P = .38).9
The BEGIN Basal-Bolus trial series evaluated use of degludec combined with bolus insulin aspart in insulin-experienced patients with T2DM (n = 992) and type 1 DM (T1DM) (n = 629) over 52 weeks.10,11 Both trials found noninferiority in A1c reduction: 1.1% (degludec) and 1.18% (glargine) in patients with T2DM and 0.4% (degludec) and 0.39% (glargine) in those with T1DM.10,11 Significantly fewer episodes of overall hypoglycemia (11.09 vs 13.63 per patient-year) and nocturnal hypoglycemia (1.39 vs 1.84 per patient-year) were found with degludec in patients with T2DM.5 Overall hypoglycemia rates were similar, though there was a 25% lower rate of nocturnal hypoglycemia with degludec in patients with T1DM.11
A meta-analysis of 7 phase 3a trials that compared degludec with glargine revealed significantly lower rates of overall, nocturnal, and severe hypoglycemia with degludec in insulin-naïve patients.12 The analysis confirmed findings of significantly lower rates of overall and nocturnal hypoglycemia with degludec in the overall T2DM population and significantly lower rates of nocturnal hypoglycemia in the T1DM population.12
In the DEVOTE trial, which included 7,637 T2DM patients at high risk for a cardiovascular event, degludec and glargine were compared on the composite primary outcome of death with a cardiovascular cause, nonfatal myocardial infarction, or nonfatal stroke. After a median of 1.99 years, the primary outcome occurred in 8.5% of degludec patients and 9.3% of glargine patients (hazard ratio, 0.91; 95% confidence interval, 0.78-1.06; P < .001 for noninferiority). Mean HbA1c level was 7.5 in both groups; severe hypoglycemia occurred more often in the glargine group (odds ratio, 0.73; P < .001 for superiority).13 Findings from the randomized, crossover SWITCH 1 and SWITCH 2 trials confirmed lower rates of symptomatic hypoglycemia with degludec compared with glargine in patients with T1DM and T2DM, respectively.14,15 No statistically significant differences in weight gain were observed in the clinical trials comparing degludec and glargine.