Evaluation and Treatment
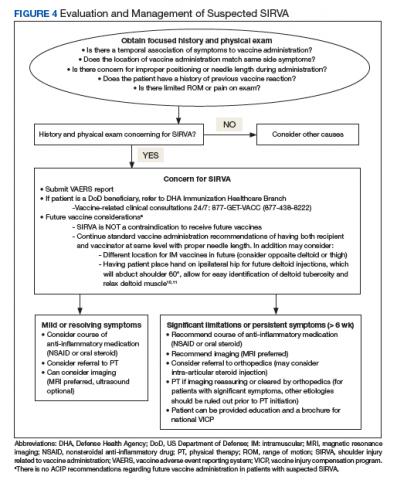
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.