Diagnosis
The diagnosis of HIT is determined by combining clinical and serologic assessment. HIT should be suspected in any patient who is in day 5 to 14 of heparin therapy and experiences a drop in platelet count of at least 50%, or in whom a new thrombotic event occurs (even if the patient is no longer receiving heparin therapy). The interpretation of all diagnostic information must be made in the context of the patient's clinical probability of HIT.15
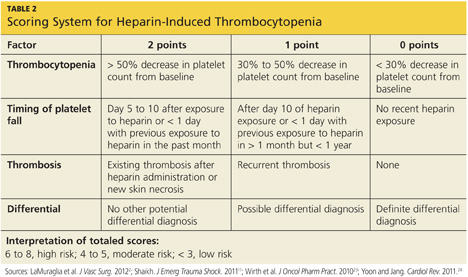
A scoring system referred to as the 4Ts (thrombocytopenia, timing of platelet fall, thrombosis or other sequelae, and test interpretation) is used to help determine the patient's probability of HIT5,21,22 (similar to the scoring strategy shown in Table 22,11,23,24).
The patient diagnosed with HIT must be positive for HIT antibodies and meet at least one additional criterion:
• A platelet count decrease of 30% to 50% below baseline, regardless of the actual value
• A venous or arterial thrombosis
• A skin lesion at the heparin injection site; and/or
• An anaphylactic reaction after IV bolus administration of heparin.15
Treatment/Management
The goal in management of HIT is to reduce the likelihood, then the severity, of thrombosis.9 Treatment should be started as soon as HIT is suspected, before laboratory confirmation.25 Treatment for HIT comprises two steps: stopping all exposure to heparin, and administering an alternative, non-heparin anticoagulant.
Discontinuation of Heparin Exposure
Stopping heparin exposure is the mainstay of treatment for HIT. This includes all potential sources of heparin exposure, including "flushes" that may be used to promote patency of central IV catheters, use of UFH-coated catheters, or addition of any heparin to total parenteral nutrition.9,25
Once discontinuation is achieved, the patient's platelet count will begin to rise within two to three days, with a return to baseline within 14 days.1 Because bleeding is rare in patients with HIT, prophylactic administration of platelets is discouraged.2,26 Platelets may be given if there is clinical evidence of bleeding; however, platelet administration has been reported to precipitate thrombosis in patients with HIT.12
If the patient's platelet count remains below baseline after 14 days of heparin abstinence, it is important for the clinician to evaluate the patient for an alternative cause of thrombocytopenia.1
Use of a Non-Heparin Anticoagulant
In addition to discontinuation of all heparin exposure, the patient must be started on a non-heparin anticoagulant, whether or not thrombosis is present.25 Forty percent to 50% of patients without thrombosis will develop a thrombosis within 30 days if alternative anticoagulation is not started.12,18,26
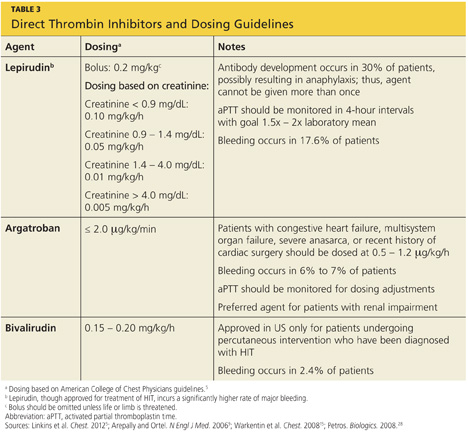
The principal choices for a non-heparin anticoagulant are the direct thrombin inhibitors (DTIs): lepirudin, argatroban, and bivalirudin.1,9,15,25,27 DTIs are the treatment of choice for patients with known or suspected HIT. The ACCP dosing guidelines for the DTIs are summarized in Table 3.9,5,15,28
The factor Xa inhibitor fondaparinux, though FDA approved for DVT prophylaxis,11 has not yet been systematically investigated for the treatment of HIT; thus, its use for this indication is considered off-label.29 However, small studies have shown no cross-reactivity between fondaparinux and PF4 antibodies.30 Due to the positive risk/benefit ratio, ease of use, and reduced need for monitoring in patients taking fondaparinux, it is considered an attractive alternative to DTIs that may receive approval in the near future.12,18,20,29
Currently, the ACCP limits its recommendation of fondaparinux use to patients with a previous history of HIT who require anticoagulation for an acute thrombotic event unrelated to HIT (grade 2C recommendation).5
The vitamin K antagonist warfarin is absolutely contraindicated in patients with HIT until the platelet count is at least 150,000/mm3, due to the risk for warfarin-induced skin necrosis and venous gangrene.9,15 If a patient is receiving warfarin at diagnosis, vitamin K (10 mg orally or 5 to 10 mg IV) should be administered.15
The patient should remain on the alternative non-heparin anticoagulant until the platelet count has stabilized at or above 150,000/mm3. Warfarin should then be started at a maximum of 5 mg/d.2,5 The non-heparin anticoagulant and warfarin should be continued until a therapeutic international normalized ratio (INR) is reached and maintained for 48 hours, with a minimum 5-day overlap of the two medications. Once the non-heparin anticoagulant is discontinued, the INR should be reevaluated for remaining within the therapeutic range, as DTIs can elevate the INR.2,5Warfarin should be continued for as long as four weeks, with frequent INR monitoring.15