Methods
From November 2011 to January 2016, the authors conducted a single-center, prospective, randomized parallel-group study in veterans undergoing elective gastrointestinal oncologic surgery. Inclusion criteria included planned esophageal, gastric, pancreatic, colorectal, or liver resections in veterans with histologically documented neoplasm of the gastrointestinal tract. Patients were excluded if they were admitted to the intensive care unit (ICU) before surgery, were receiving steroids or other immunosuppressive medications, had a recent hospital admission for pulmonary, cardiac, or renal disease, or were exhibiting signs or symptoms of infection or sepsis, including elevated white blood cells (WBC) > 10,000/mL or a temperature > 37.7° C.
The study was approved by the research and development committee and the institutional review board at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. The clinicaltrials.gov identifier for the study was NCT01471743.
Nutrition Formula
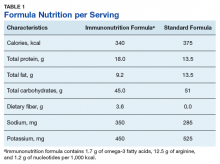
Subjects were randomized into 2 oral supplement groups: immunonutrition group (ING) patients received immunonutrition, and standard nutrition group (SNG) received a standard formula (Table 1).
Each participant received the supplement and were instructed to drink 3 servings per day (750 mL/d) for 5 days before their surgery.Study Procedures
All veterans with planned gastrointestinal surgeries were evaluated in the JAHVH general surgery clinic. Veterans meeting the inclusion criteria were invited to participate in the study, and informed consent was obtained. A research randomizer program assigned subjects to the groups to reach equal 1:1 randomization. Enrolled participants were provided their randomized supplement (unblinded) in the general surgery clinic and instructed on the amount of supplement to consume and date to begin taking the supplement. Participants were instructed to continue with their normal diet in addition to the supplement. No additional nutrition education was provided. Participants were asked to keep track of their daily supplement intake. Patients in both groups also used preoperative bowel preparations when indicated.
At the time of enrollment, presurgical comorbidities, anthropometric data, and nutrition status parameters were obtained. Postoperatively, study personnel interviewed each patient about formula consumption and tolerance. Thirty days postoperatively, patient demographics, surgical characteristics (eg, surgery, operative time, blood loss), nutrition risk screening (NRS 2002) score, diet/enteral orders, days spent NPO, days in the hospital or in the ICU, and complications (eg, wound infection, abscess, sepsis, pneumonia, urinary tract infection, intestinal fistula, ileus, or anastomotic leakage) were collected from the electronic health record.
Statistical Analysis
The primary outcome measure was overall postoperative complication rate and postoperative infection rate. Based on reviews of similar studies available at the time of protocol development, it was assumed that a postoperative infection rate of 38% in the SNG and 15% in the ING would indicate treatment efficacy. A sample size of 54 patients in each group would provide a Type I error level α = .05 and a power of 80%. A total of 108 patients enrolled in the study. Chi-square analysis was used to determine this primary outcome measure.
Secondary outcomes (mean number of complications, hospital days, NPO (nothing by mouth) days, and ICU days) were evaluated with Mann Whitney U test because of violation of assumptions for the t test. All P values were 2-tailed and statistical significance was accepted at P < .05 with clinical significance accepted at P < .10. Analysis for intention to treat (ITT) and per protocol are provided for outcome measures. For the ITT analysis, multiple imputation (last observation carried forward) was used. Sensitivity analysis found that the data were missing at random. SPSS software version 21.0 (Chicago, IL) was used for statistical analysis.
Results
During the study period, 137 patients were assessed for eligibility (Figure).
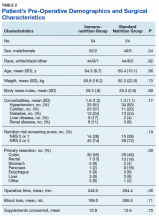
An ITT as well as a per-protocol analysis was reviewed by the authors and presented to the hospital nutrition committee before making protocol decisions. A full review of all enrolled study subjects (including those who did not receive actual supplementation) was evaluated for factors that could influence bias from dropped treatment. However, the authors also wanted to evaluate treatment efficacy for only those who received supplementation; therefore, a per protocol analysis was reviewed. Both analyses are included. For the ITT analysis, 54 subjects in each group were analyzed. Six participants in the ING and 7 in the SNG did not receive surgical intervention, respectively. As a result, 47 SNG and 48 ING participants were included in the per-protocol analysis.The sample was predominately white and male, which is consistent with the veteran population. There were no statistical differences for baseline patient or surgical characteristics between the groups (Table 2).
The mean (SD) number of comorbidities was slightly higher in the ING compared with those of the SNG, 1.6 (1.2) vs 1.3 (1.1), respectively. In addition, there was a trend (P = .06) of longer operative time in the SNG (mean 294.4 minutes) compared with that of the ING (mean 249.5 minutes). There was no difference in supplemental intake between the groups and an overall adherence rate of 86% in both groups (Table 2). A total of 41 participants in the ING consumed ≥ 10 servings in 5 days vs 35 in the SNG.There was a significant difference (P = .09) in the surgical procedures completed. There was only 1 pancreatic surgery completed in the ING and 9 pancreatic surgeries completed in the SNG.