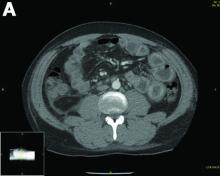
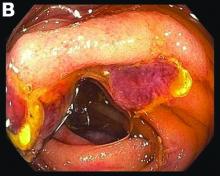
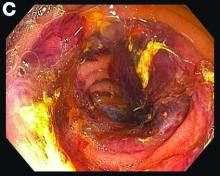
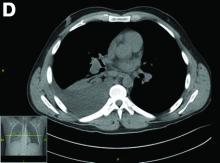
Erosive protein-losing enteropathy secondary to disseminated histoplasmosis
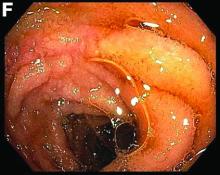
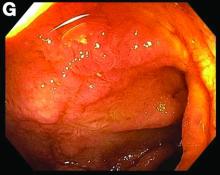
This patient was treated with amphotericin B and transitioned to oral itraconazole with frequent blood level monitoring to ensure absorption. His symptoms improved gradually. Small-bowel enteroscopy 3 weeks after presentation showed a normal duodenum and healing, superficial ulcers in the proximal jejunum (Figure F, G). Blood albumin levels had recovered to 3.1 g/dL (normal, 3.5–5.0 g/dL).
Protein-losing enteropathy (PLE) is a rare syndrome characterized by loss of serum proteins in the gastrointestinal (GI) tract, resulting in significant hypoproteinemia and consequent edema.1 PLE can also result in ascites, pleural and pericardial effusions, and, in prolonged cases, malnutrition. There are a variety of causes of PLE that can be broadly grouped into erosive GI disorders, disorders of increased GI mucosal permeability, and disorders of increased interstitial pressure. The clinical presentation depends on the underlying etiology, but commonly includes generalized edema owing to hypoproteinemia and resulting reduced oncotic pressure. GI symptoms are not frequently observed. The initial step in evaluating a patient with symptoms concerning for PLE is to rule out more common causes of hypoproteinemia, such as renal or hepatic disease, and malnutrition. To confirm enteric protein loss, alpha 1-antitrypsin clearance with a 24-hour stool collection is commonly and reliably used. Treatment of PLE is centered on treating the underlying cause while monitoring and treating malnutrition, including micronutrient deficiencies.
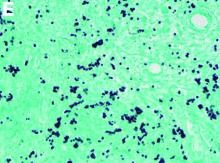
Fungal infections are a rare cause of PLE, but important to recognize as a potential complication of tumor necrosis factor–therapy, because these medications are commonly used for a variety of autoimmune diseases.2 Although histoplasmosis is an uncommon cause of GI inflammation, disseminated histoplasmosis causing PLE has been previously reported.3 In our patient, Histoplasma capsulatum infection caused diffuse GI ulcers, which allowed protein loss in the GI tract (erosive PLE). Antifungal treatment resulted in healing of intestinal ulcers and correction of hypoalbuminemia, thereby confirming the diagnosis of PLE and obviating the need for a confirmatory alpha 1-antitrypsin clearance study.
References
1. Umar SB, DiBaise JK. Protein-losing enteropathy: case illustrations and clinical review. Am J Gastroenterol. 2010;105:43-9.
2. Tsiodras S, Samonis G, Boumpas DT. et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-94.
3. Kok J, Chen SC, Anderson L, et al. Protein-losing enteropathy and hypogammaglobulinaemia as first manifestations of disseminated histoplasmosis coincident with Nocardia infection. J Med Microbiol. 2010;59:610-3.