INTRODUCTION
Hodgkin lymphoma, previously known as Hodgkin’s disease, is a B-cell malignancy with unique pathological and epidemiological features for which highly effective therapies exist. The disease is characterized by the presence of mononuclear and multinucleate giant cells called Hodgkin and Reed-Sternberg (HRS) cells. 1
Hodgkin lymphoma is unique compared to other B-cell lymphomas because of the scarcity of the malignant cells in the tumor tissue. The HRS cells usually account for only 0.1% to 10% of the cells in the affected tissues, and the HRS cells induce accumulation of nonmalignant lymphocytes, macrophages, granulocytes, eosinophils, plasma cells, and histiocytes, which constitute more than 90% of tumor cellularity. 2 Although the disease was first described by Sir Thomas Hodgkin in 1832, in part because of this unique histopathology, not until 1991 was it conclusively demonstrated that HRS cells are in fact monoclonal germinal center–derived B-cells. This article reviews management and frontline treatment options for limited-stage classical Hodgkin lymphoma and nodular lymphocyte predominant Hodgkin lymphoma. Treatment of advanced stage and relapsed/refractory Hodgkin lymphoma will be discussed in a separate article.
EPIDEMIOLOGY
Hodgkin lymphoma accounts for 0.5% of all malignancies and 11.7% of all lymphomas among adults in the United States. 3 The incidence of Hodgkin lymphoma has been steadily increasing over the past 4 decades and was estimated to be 8260 cases in the United States in 2017, with a slight male predominance. Hodgkin lymphoma is expected to cause 1070 deaths in 2017, accounting for 0.2% of all cancer deaths. 3 First-degree relatives of patients with Hodgkin lymphoma have a 3- to 9-fold increased risk of having the disease compared to the general population, 4 and monozygotic twin siblings of Hodgkin lymphoma patients have a greatly increased risk for developing the disease—up to 100-fold—compared to normal cohorts. 5 The incidence is highest among Caucasians, African Americans, and Hispanics, and lower in Asians and American Indians. 3 Hodgkin lymphoma incidence shows a bimodal peak distribution, with 1 peak between the ages of 15 and 44 years, and another peak after age 65 years. 6
ETIOLOGY/PATHOGENESIS
The cause of Hodgkin lymphoma is unknown. Epstein-Barr virus (EBV) infection is present in up to 40% of Hodgkin lymphoma cases, suggesting a role of this virus in the pathogenesis of some cases. The risk of EBV-positive Hodgkin lymphoma was found to be higher following an episode of infectious mononucleosis, while the risk of EBV-negative Hodgkin lymphoma remained unchanged. 7 The incidence of Hodgkin lymphoma is 5 to 14 times higher in HIV-infected patients than in noninfected patients. 8 It is not considered an AIDS-defining illness, but has become more frequent with the growth and aging of the HIV-positive population. 9,10 Hodgkin lymphoma patients with HIV typically have CD4 lymphocyte counts greater than 200 cells/μL, 11 with the incidence of Hodgkin lymphoma actually declining with lower CD4 lymphocyte counts. 12 HIV-related Hodgkin lymphoma tends to have an aggressive course, with high rates of EBV positivity. 13 The incidence of Hodgkin lymphoma is 1.8 times higher among smokers, and the risk appears to increase with duration of smoking. 14,15
The cell of origin of Hodgkin lymphoma, while long suspected to be the HRS cell, remained unproven until the 1990s when micro-dissection and single-cell polymerase chain reaction techniques allowed for confirmation that the HRS cell was in fact a monoclonal germinal center derived B cell. 16,17 These HRS cells lack immunoglobulin due to defective transcription regulation and not due to crippling mutations. 18,19 The cellular infiltrate in Hodgkin lymphoma appears to play a decisive role in allowing the HRS cells to survive by providing an environment that suppresses cytotoxic immune responses as well as by providing cellular interactions and cytokines that support their growth and survival. The extensive inflammatory infiltrate in classical Hodgkin lymphoma is comprised of T helper 2 (Th2) and regulatory T cells and lacks T helper 1 (Th1) cells, CD8 cytotoxic T cells, and natural killer cells. 20 The HRS cells escape apoptosis by several mechanisms which include latent EBV infection and constitutive nuclear factor (NF)-kB pathways, as well as other deregulated signaling pathways that promote survival, such as EBV nuclear antigen 1 (EBNA1) protein, EBV latent infection membrane protein 1 (LMP1), and LMP2. 21,22
Genetic alterations in the 9p24 locus which encodes PD-L1/PD-L2 are nearly universally present in classical Hodgkin lymphoma and are now considered a disease-defining feature. 23
PATHOLOGIC CLASSIFICATION
According to the 2008 World Health Organization (WHO) classification, Hodgkin lymphoma has 2 clearly defined entities: classical Hodgkin lymphoma (cHL), which accounts for approximately 95% cases, and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), which accounts for the remaining cases. 24 These 2 entities differ in their clinical, pathological, and biological features, which in turn affect prognosis and treatment options. Classical Hodgkin lymphoma is characterized by a paucity of HRS cells surrounded by a background of mixed inflammatory infiltrate comprised of histiocytes, small lymphocytes, eosinophils, neutrophils, plasma cells, fibroblasts, and collagen. Depending on the particular combinations of these elements and the specific features of the neoplastic cells, cases can be subclassified into several cHL subtypes: the nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted types. 25
The diagnosis of cHL is made based on a combination of morphology of HRS cells and the other cells infiltrating the tissue, combined with immunohistochemical staining. Because of the rare nature of the malignant (clonal) cell in Hodgkin lymphoma specimens, flow cytometry is generally of little value. The HRS cells in cHL are CD30-positive and CD45 negative in virtually all cases, and CD15-positive in 85% of cases. 26 B-cell antigens are typically negative except for CD20, which is positive in about 20% cases. 27
Nodular sclerosis Hodgkin lymphoma (NSHL) is the most common subtype of cHL, accounting for 65% to 75% of cases. It is common among young adults and tends to involve the mediastinal, supraclavicular, and cervical lymph nodes. NSHL is characterized by the presence of collagen bands that divide the lymphoid tissue into circumscribed nodules. This subtype usually presents as stage I or II disease, typically with neck and/or mediastinal disease, and evidence of EBV infection is present in approximately 10% to 40% of North American cases. 7 Patients diagnosed with NSHL generally have a very good prognosis.
Mixed cellularity Hodgkin lymphoma (MCHL) constitutes about 20% to 25% of cHL cases. It affects a somewhat older population, with a median age at diagnosis of 38 years. The typical bimodal age distribution is not seen with MCHL. MCHL has a male predominance (70%), and is more frequent in HIV-infected patients (70% of whom also have EBV infection). Lymphoid tissues have classic HRS cells and significant inflammatory infiltrates. Approximately 50% of patients with MCHL present as stage III or IV with abdominal lymphadenopathy or splenic involvement, and B symptoms are frequent. 24
Lymphocyte-rich Hodgkin lymphoma (LRHL) is uncommon, accounting for only 3% to 5% of cases of cHL. 28 The disease usually presents at an older age and has a 2:1 male predominance. HRS cells are commonly seen and a large number of reactive lymphocytes are also present. Although on the basis of morphology and immunohistochemistry LRHL belongs to the cHL group, clinically it more closely resembles LPHL. Patients usually present at early stage and rarely have B symptoms. LRHL carries an excellent prognosis, with a greater than 90% PFS after 5 years. 23,29
Lymphocyte-depleted Hodgkin lymphoma (LDHL) is the least common form of cHL, accounting for less than 5% of cases. Many cases previously placed in this category are now recognized as diffuse large B-cell lymphoma (DLBCL), anaplastic large-cell lymphoma (ALCL), or NSHL with lymphocyte depletion. 30 HRS cells are frequently seen, but reactive inflammatory cells are relatively sparse. EBV infection is seen in up to 90% of cases, commonly associated with HIV-infected individuals. Advanced-stage and symptomatic disease are more common. Prognosis is slightly worse compared to other categories.
NLPHL accounts for approximately 5% of cases of Hodgkin lymphoma. It has a unimodal age distribution, with the peak incidence in the fourth decade, and male predilection of 3:1. 28 NLPHL is characterized by large primary lymphoid follicles, with polytypic small B lymphocytes and extensive meshworks of follicular dendritic cells. The lymphocytic/histiocytic (L and H), or “popcorn,” cells scattered within the nodules differ from classic HRS cells, both in their morphology and in their biochemical profile, being frequently negative for CD15, CD30 and for the EBV genome, and usually positive for B-cell antigens such as CD20, suggesting that L and H cells may be immunoglobulin-synthesizing monoclonal B cells. CD45 is also typically positive in NLPHL, in distinction from cHL. NLPHL has an indolent course compared to cHL, and long-term survival is common. 19,31 NLPHL commonly presents with limited-stage disease. NLPHL may eventually transform into a more aggressive lymphoma, such as diffuse large B-cell lymphoma (including centroblastic, immunoblastic, or T-cell/histiocyte–rich subtypes), at a rate of 4% to 12%. This can occur even 15 to 20 years after the initial diagnosis of NLPHL. 32,33 In a recent large retrospective study of 222 patients with NLPHL, the rate of transformation to DLBCL was 7.6%, with a median time to transformation of 35 months. Overall survival was not adversely affected in patients undergoing transformation compared to those without transformation. 34
PRESENTATION
Classical Hodgkin lymphoma usually presents with asymptomatic mediastinal or cervical lymphadenopathy. Half of patients present with stage I or stage II disease. 35 A mediastinal mass is seen in most patients with NSHL, at times with bulky disease, with “bulky” defined as a mediastinal mass measuring one-third or more of the maximal thoracic diameter on chest x-ray, or 10 cm on computed tomography (CT) scan. Systemic symptoms, or "B" symptoms—fevers (> 38 °C), drenching night sweats, and unexplained weight loss (> 10% of baseline body weight over the preceding 6 months or less)—are detected in approximately 25% of patients. Between 10% and 15% will have extranodal disease, most commonly involving lung, bone, and liver. NLPHL usually presents with limited-stage disease without B symptoms; it typically has a more indolent presentation and clinical course than cHL.
INITIAL EVALUATION AND STAGING
The initial workup includes a complete blood count (CBC), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), and chemistry studies to evaluate renal function and liver function. Fine-needle aspiration will usually fail to identify the infrequent HRS cells, and instead only reveal the reactive background of inflammatory cells. Generous (large gauge) core needle biopsies may provide diagnosis effectively in some cases, but in general, an excisional lymph node biopsy is preferred to ensure an accurate diagnosis and avoid the need for repeated biopsy procedures. In cases where an excisional biopsy would be difficult or risky, a core needle biopsy procedure is a reasonable first step, with the understanding that a subsequent surgical procedure may still be necessary.
Baseline imaging includes CT scans of the neck, chest, abdomen, and pelvis. Use of positron emission tomography (PET) scanning is now standard in the initial evaluation and assessment of treatment response in Hodgkin lymphoma. 36 Due to the increased sensitivity of PET or PET/CT scan, additional lesions may be identified that were not seen on conventional CT scans. This will alter the staging, and potentially the treatment plan, in up to 25% to 30% of patients. 37,38 PET/CT scan performed during initial evaluation also facilitates optimal interpretation of post-therapy PET/CT scans and is therefore strongly encouraged as a part of the initial staging evaluation. 39
Recent studies have shown that bone marrow biopsy is not routinely needed in the initial staging of cHL. A study of 454 patients concluded that bone marrow biopsy would not have altered the stage in any stage I or II patients. It was further concluded that overall treatment strategy would not have been altered for any of the patients. 40 Based on this study and others, it is now clear that FDG-PET has a high sensitivity, and when PET scan is negative (in the bone marrow and skeleton), a bone marrow biopsy provides little additional value. For patients with significant cytopenias, a bone marrow biopsy is reasonable. Such patients may benefit from a bilateral biopsy, which increases the probability of demonstrating bone marrow involvement by 16% to 33%. 41,42 Techniques such as staging laparotomy and lymphangiography are now considered obsolete.
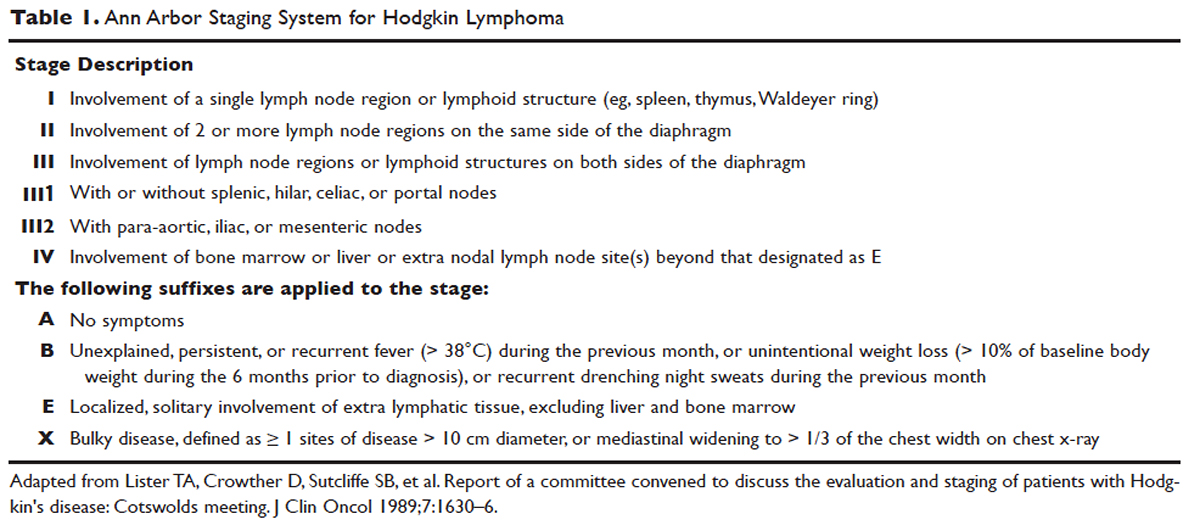
Hodgkin lymphoma is staged according to the Ann Arbor staging system ( Table 1 ). The original Ann Arbor staging was published in 1971, 43 and in 1989 the “Cotswold modifications” extended the definitions of stage IV disease and the suffix “X” was added to denote bulky disease. 44 Both systems were developed for the staging of Hodgkin lymphoma, but are now used for staging non-Hodgkin lymphoma as well.
PROGNOSTIC FACTORS
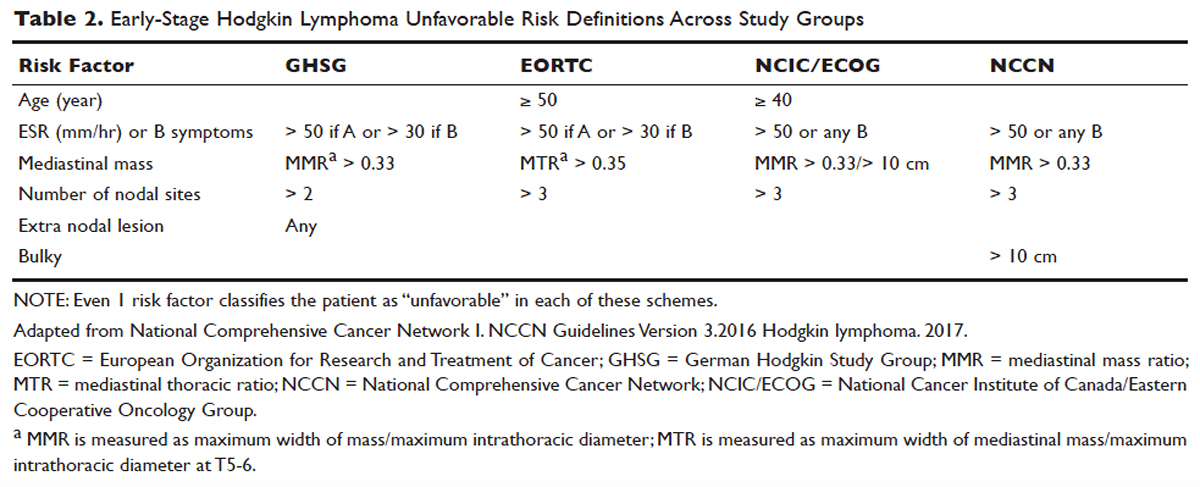
For the purposes of prognosis and selection of treatment, Hodgkin lymphoma is commonly classified into early-stage favorable, early-stage unfavorable, and advanced stage. Early-stage Hodgkin lymphoma refers to patients with Ann Arbor stage I or stage II disease. With early-stage Hodgkin lymphoma, the prognosis varies significantly based on factors such as the presence of B symptoms, elevated erythrocyte sedimentation rate ([ESR] > 50 mm/hr), number of nodal sites involved, older age, and a large mediastinal mass. For this reason, most clinical trials to evaluate treatment strategies for early-stage Hodgkin lymphoma are based on various combinations of these risk factors. The definition of early-stage unfavorable Hodgkin lymphoma varies across different clinical trial study groups, and it is important to understand the definition in interpreting the results of these trials ( Table 2 ).45,46
In the German Hodgkin Study Group (GHSG) trials, early-stage Hodgkin lymphoma is stratified into a high risk (“unfavorable”) group defined by any of the following: a large mediastinal mass (one third of maximum thoracic diameter), extra-nodal disease, 3 or more nodal areas, and an ESR of > 50 mm/hr in asymptomatic patients or > 30 mm/hr in patients with B symptoms. Low-risk (“favorable”) patients lack all of these factors. 47 The European Organization for Research and Treatment of Cancer (EORTC) defines the unfavorable prognostic group as older than 50 years of age, large mediastinal adenopathy (maximum width on a chest radiograph of at least one third of the internal transverse diameter of the thorax at the level of T5 through T6 or any mass of ≥ 10 cm in the largest dimension), an ESR of 50 mm/hr and no B symptoms, or with an ESR of 30 mm/hr in those who have B symptoms, and/or 4 or more regions of involvement. 48 The National Cancer Institute of Canada (NCIC) Clinical Trials Group and the Eastern Cooperative Oncology Group (ECOG) define high-risk groups as presence of B symptoms, bulky disease with a mediastinal mass width of at least one third of the maximum chest wall diameter, or any mass greater than 10 cm, and patients with intra-abdominal disease. 49,50
Gene-expression profiling in Hodgkin lymphoma has identified a gene signature of tumor-associated macrophages that was able to identify patients with a higher risk for primary treatment failure. In an independent cohort of patients, an increased number of CD68-positive macrophages was correlated with inferior outcomes. 51,52 Studies such as these underscore the importance of the tumor “microenvironment” (ie, the nonmalignant cells within a tumor) in determining the overall clinical behavior of a malignancy. While quantification of CD68-positive macrophages has potential to be applied to routine clinical practice, prospective data using CD68 as a tool for risk-adapted therapy is lacking.
Genetic alterations and amplifications in the 9p24.1 locus have recently been found to be a defining genetic feature of cHL. Amplification of 9p24.1 has been associated with unfavorable outcomes. Amplification of 9p24.1 (which includes the loci encoding the PD-L1 and PD-L2 genes) is more common in patients with advanced stage disease and is associated with shorter PFS. 23
A recent study attempted to integrate several different prognostic factors in cHL patients who were treated with ABVD (adriamycin [doxorubicin], bleomycin, vinblastine, and dacarbazine) and underwent an interim PET (iPET) scan after 2 cycles of ABVD. Focusing on those with a negative iPET scan, it was found that expression of CD68 and PD-1 in microenvironment cells, and STAT1 negativity in HRS cells identified a subset of PET-2 negative patients with a 3-year PFS significantly lower than that of the remaining PET-2 negative population (64% versus 95%). The algorithm correctly predicted the response to treatment in more than half of the patients who had relapse or disease progression despite a negative PET-2 scan. It therefore appears feasible, using tissue biomarkers at diagnosis, to identify patients at increased risk for poor outcome, even if the iPET scan is negative. 53
ROLE OF PET/CT IN ASSESSMENT OF RESPONSE TO THERAPY
PET/CT has been increasingly used for response assessment at various stages in lymphoma in recent years. Almost all types of lymphomas are fluorodeoxyglucose (FDG) avid; however, Hodgkin lymphoma is FDG avid in 97% to 100% of cases. In 2009, a 5-point scale was developed to score PET images with regard to treatment response, either partway through treatment (iPET) or at the end of therapy. 54 It was recommended as the standard reporting tool at the First International Workshop on PET in Lymphoma in Deauville, France, in 2009, and is thus now referred to as the Deauville score. A score of 1 is given if there is no uptake, 2 if the uptake ≤ mediastinum, 3 if > mediastinum but ≤ liver, 4 if uptake moderately higher than liver, 5 if uptake is markedly higher than liver and/or new lesions. X designates new areas of uptake unlikely to be related to lymphoma. In most trials, a score of 1 or 2 is considered a complete response and a score of 4 or 5 is considered a treatment failure. A score of 3 is sometimes considered a complete response, depending on the study. The Deauville criteria have been widely used in newer clinical trials utilizing response-adapted treatment as defined by PET response. PET/CT is recommended for staging and restaging at the end of therapy, in clinical practice, and clinical trials. Interim PET/CT scan, while commonly performed in clinical practice, is only recommended if the results will alter therapy (eg, if that information will result in the clinician omitting radiation therapy [RT] or altering the chemotherapy plan).
Early studies of iPET showed that achieving PET negativity early in the course of treatment was strongly associated with PFS and overall survival. 55 Subsequent studies confirmed the importance of achieving a negative iPET. As a result, considerable efforts have been put into designing response-adapted treatment approaches using iPET (see Treatment section), with some of these approaches now being listed in the National Comprehensive Cancer Network (NCCN) guidelines and being used in standard practice.
TREATMENT
EVOLUTION OF TREATMENT
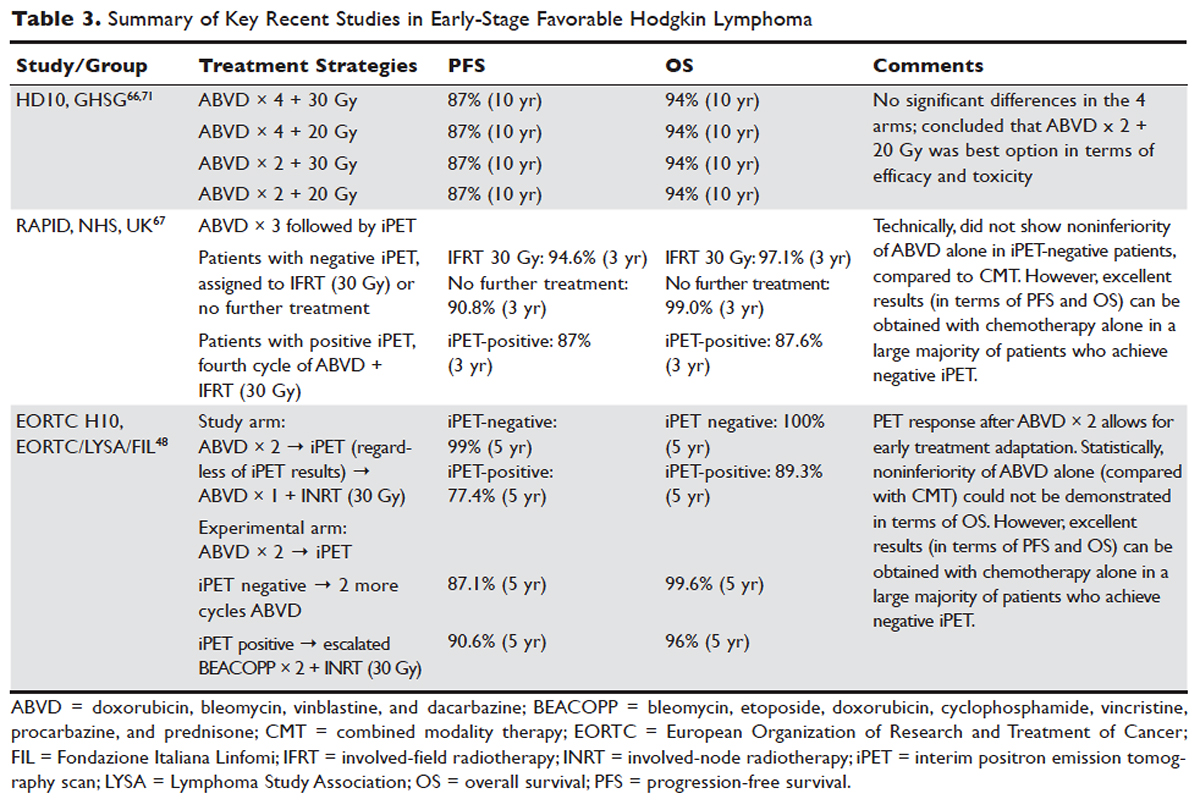
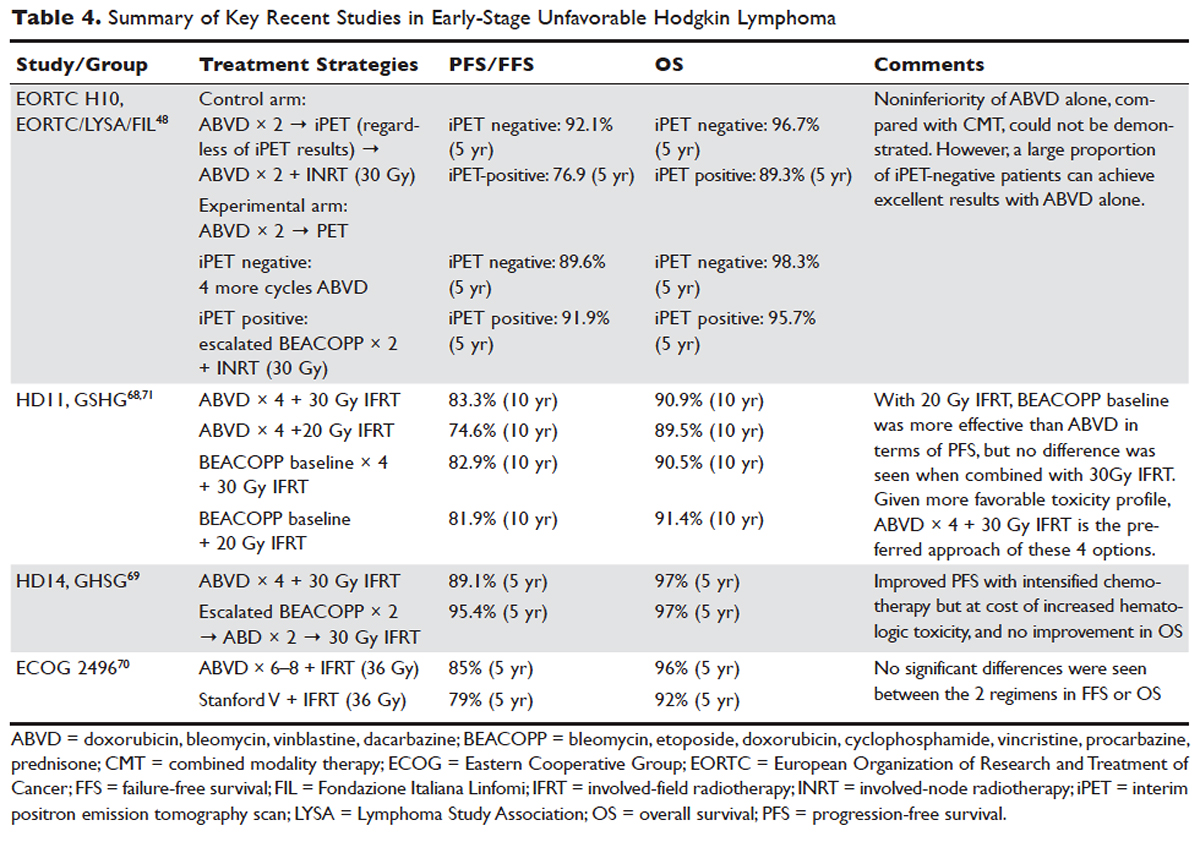
The treatment of Hodgkin lymphoma has evolved over the past century, starting with the discovery of RT as effective treatment in the early 20th century. Long-term survival of patients with Hodgkin lymphoma treated with involved-field radiation therapy (IFRT) was first reported in the 1960s. 56,57 Outcomes improved further with the introduction of combined modality treatment (CMT) using chemotherapy and RT, with the overall 5-year relative survival for patients with Hodgkin lymphoma (all stages) treated in 2006–2012 being 85.4% to 87.3%. 3 Since the majority of patients are now cured with modern therapy, treatment-related complications have become an important competing cause of mortality. Recent studies have therefore focused on maintaining efficacy while reducing toxicities, and refining the process of selecting patients who might benefit from more aggressive therapy. While RT was the first treatment modality shown to be curative for Hodgkin lymphoma, 56,57 multiple subsequent studies showed that CMT is superior to RT alone in terms of relapse-free survival. 58–63 In the GHSG H8-F trial, the estimated 5-year event-free survival and overall survival rates were significantly higher after 3 cycles of MOPP-ABV (mechlorethamine, vincristine, procarbazine, and prednisone combined with doxorubicin, bleomycin, and vinblastine) plus IFRT than after subtotal nodal radiotherapy alone. The 10-year overall survival estimates were 97% and 92%, respectively ( P = 0.001). 64 As a result, CMT replaced RT alone as the standard of care for limited-stage Hodgkin lymphoma. However, for elderly or infirm patients, or those with other comorbidities making them poor chemotherapy candidates, RT alone may be a very reasonable option. 65 More recently, an increasing body of evidence has accumulated to support the use of chemotherapy alone in early stage cHL. This literature has consistently shown that omission of RT is associated with a modest increase in relapse, without a clear compromise in long-term overall survival. For some patients, the trade-off in terms of avoiding radiation (and the associated late effects) may be worth the small increase in relapse risk, since long-term survival does not appear to be substantially worse with chemo-therapy alone. Table 3 and Table 4 provide a summary of recent key studies which have defined treatment options for early-stage cHL. 48,66–71
EARLY-STAGE NLPHL
NLPHL usually presents with limited-stage disease without B symptoms and has an indolent course with a slightly better prognosis compared to cHL. 72 Due to the rarity of the disease, treatment guidelines are mostly based on retrospective analyses from single or multi-institution studies or subgroup analyses, often with relatively short follow-up. These studies must be interpreted with caution because of the possibility of inaccuracies in the pathologic diagnosis, small sample sizes, and selection bias. Treatment options for limited-stage NLPHL include observation, single-agent rituximab, IFRT (or involved-site radiation therapy [ISRT]) alone, or CMT. 46
Historically, patients with limited-stage NLPHL have been treated with RT alone, with 80% to 85% PFS and 85% to 95% overall survival rates. 73–75 Patients who relapse or progress after RT in general can successfully undergo salvage therapy. 74 In one study, rates of PFS and overall survival were similar among patients who had limited-field, regional-field, or extended-field RT, 75 indicating that IFRT is preferred. Studies comparing RT alone and CMT are limited. The GHSG HD7 trial included a subset of NLPHL patients, with a trend towards improved freedom from treatment failure (96% versus 83%) favoring CMT. This, however, did not translate into improved overall survival. 47 A retrospective analysis of the British Columbia Cancer Agency database compared patients with limited-stage NLPHL treated with RT alone to patients who received 2 cycles of ABVD followed by RT. A significant improvement in PFS (91% versus 65%) and overall survival (93% versus 84%) was seen, favoring CMT. 76
Chemotherapy alone is not recommended for limited-stage NLPHL since studies evaluating chemotherapy alone are quite limited and indicate relatively high rates of treatment failure. Given that the malignant cells in NLPHL are CD20-positive, single-agent rituximab has also been studied in this disease, including a study as frontline therapy in limited-stage patients. In this phase 2 trial in newly diagnosed patients with stage IA disease, an overall response rate (ORR) of 100% was seen, with an 85% complete response (CR) rate. 77 At 3 years, overall survival was 100% and PFS was 81%, indicating that the responses with single-agent rituximab are less durable than those with RT.
Advani et al evaluated rituximab followed by observation versus rituximab (R) followed by maintenance rituximab (MR) for 2 years in 39 new or previously treated patients. At 4 weeks the ORR was 100% (with CR in 67%, and partial response in 33%). At a median follow up of 9.8 years for R alone, and 5 years for R+MR, median PFS was 3 and 5.6 years, respectively ( P = 0.26). Estimated 5-yr PFS and overall survival in patients treated with R versus R+MR were 39.1% and 95.7% versus 58.9% and 85.7%, with Pvalues of 0.26 (PFS) and 0.38 (overall), respectively. Maintenance rituximab therefore appears to prolong remission, although the results did not quite reach statistical significance. 78 Even though rituximab does not appear to be curative in NLPHL, it is a reasonable option for patients with early-stage NLPHL who are not good candidates for definitive RT. Whether combining rituximab with RT or CMT might further improve outcomes in early-stage NLPHL has not yet been determined.
In children, surgery alone may lead to long-term remission or possibly cure of limited-stage NLPHL. In a European multicenter retrospective study, 58 patients underwent surgery for limited-stage NLPHL. Among the 51 patients who achieved complete remission following surgery, 67% remained progression-free and 100% were alive at a median follow up of 43 months. 79 In adults, there is no data to support surgical treatment alone for NLPHL. Finally, observation may be a reasonable option in elderly or infirm patients for whom NLPHL is unlikely to affect life expectancy. For younger patients, given the excellent outcome with modern therapy and the long-term risk of transformation of NLPHL into an aggressive non-Hodgkin lymphoma, observation is generally not recommended.
The NCCN recommends RT (ISRT or IFRT, 30–36 Gy) as the preferred treatment for stage IA and IIA non-bulky NLPHL. In patients with stage IA disease with complete excision of solitary nodule, observation may be appropriate. A course of chemotherapy with ISRT with or without rituximab is recommended for patients with stage IB or IIB disease, or patients with stage IA or IIA bulky disease.
FIRST-LINE TREATMENT OF LIMITED-STAGE CHL
Early-Stage Favorable cHL
There is lack of consensus regarding the ideal treatment approach for patients with early-stage favorable cHL. However, there are several excellent options available, with overall survival rates exceeding 90%. Most of these regimens involve CMT, although some chemotherapy-alone approaches have been evaluated as well. Concurrent with the demonstration of excellent long-term remission rates with CMT, it became apparent that the long-term survival and quality of life of these patients is determined in large part by the risk of serious (and potentially fatal) treatment-related toxicities. Such toxicities consist primarily of secondary malignancies and cardiovascular events, and can continue to cause significant morbidity and mortality even 2 to 3 decades after treatment. 80–82 As a result, treatment decisions for these patients are complicated and require balancing efficacy against risk of late complications.
In the United States, until recently, CMT was generally considered the standard of care, with robust long-term data regarding efficacy. The most commonly used regimen has been ABVD for 2 to 4 cycles followed by IFRT. In some German studies, escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) has been used, but this is not a general standard of care in the United States for early-stage patients.
More recent data suggests that the rate of serious late complications in Hodgkin lymphoma patients is decreasing, likely due to less extensive radiation fields, lower radiation doses, and a movement away from the MOPP regimen to ABVD. 83,84 For patients who meet the “favorable” criteria set forth in the GHSG HD10 trial (see Table 2), 2 cycles of ABVD followed by 20 Gy of IFRT is an attractive option, with efficacy preserved and a low anticipated rate of late effects. 66 With this approach, and with long-term (10 years) follow up, all 4 arms had similar PFS (87%) and overall survival (94%), whether 2 or 4 cycles of ABVD were given. When the effects of 20-Gy and 30-Gy doses of RT were compared, there were also no significant differences in freedom from treatment failure or overall survival. Adverse events and acute toxic effects of treatment were most common in the patients who received 4 cycles of ABVD and 30 Gy of RT. 66,71
In recent years, in an attempt to reduce late effects further, regimens consisting of chemotherapy alone have been investigated. In a study by Meyer et al, at 12 years the rate of overall survival was 94% among those receiving ABVD alone, as compared with 87% among those receiving subtotal nodal RT; the rates of freedom from disease progression were 87% and 92% in the 2 groups; and the rates of event-free survival were 85% and 80%, respectively. 50 However, it is important to note that this study did not include a CMT arm for the early favorable patients, and did not utilize modern RT techniques. Nevertheless, this early study and others 60 suggested that chemotherapy alone may be a reasonable option for some early-stage cHL patients, particularly for patients who are felt to be at increased risk for late toxicities from RT. As a result, additional studies have been conducted evaluating CMT versus chemotherapy alone for early-stage cHL. Many of these studies have incorporated interim PET/CT scan to develop a response-adapted approach to decide which patients are least likely to benefit from RT.
The HD-13 study was a follow-up study for HD-10, looking at deletion of bleomycin, dacarbazine, or both from the ABVD backbone. The ABD arm was closed early, because of an excess rate of treatment failure. Among the 1243 patents assigned to either the ABVD or AVD arm at 5 years of follow-up, there was 4.3% difference in PFS. This study was not able to demonstrate that 2 cycles of AVD was noninferior to 2 cycles of ABVD, each followed by 30 Gy IFRT, even though there was no difference in all 4 groups. It confirmed 2 cycles of ABVD as the preferred regimen in early favorable Hodgkin lymphoma, when CMT is the plan of care. However, for patients over age 60 to 65 years, or those with underlying cardiac or pulmonary comorbidities, bleomycin has significant risk of toxicity. In that setting, AVD is a safer option, with only a very modest decrease in 5-year PFS.
Based on the observation that iPET scan is highly predictive of outcome in Hodgkin lymphoma, 55,85 several trials have employed the use of an iPET scan to guide therapy. It is hoped that such studies will lead to new PET-directed treatment algorithms in which patients who require more aggressive therapy (eg, with CMT, or escalated BEACOPP) can be identified, and the remaining patients can be safely treated less aggressively (eg, with chemotherapy alone).
In the EORTC H10 trial, performed to evaluate treatment adaptation on the basis of iPET scan results in stage I and II Hodgkin lymphoma, a control arm received standard combined modality treatment (3 or 4 cycles of ABVD with INRT) irrespective of PET scan results. In the experimental arm, patients with a negative PET scan after 2 cycles of ABVD continued with 1 or 2 cycles of ABVD and did not receive RT. The iPET-positive patients received either standard treatment with ABVD plus INRT or escalated BEACOPP plus INRT. The iPET-negative patients received either ABVD only or ABVD plus INRT. The final results of this study, published recently, showed that in the iPET-positive patients the 5-year PFS was improved from 77.4% with standard ABVD plus INRT to 90.6% with escalated BEACOPP plus INRT ( P = 0.002). In iPET-negative patients, 5-year PFS in the favorable group was 99% versus 87.1% in favor of ABVD plus INRT. The H10 study suggested that PET results after 2 cycles of ABVD can be integrated into treatment planning, In iPET-negative patients, the study was technically not able to demonstrate the noninferiority of the ABVD only regimen, owing to a higher risk of relapse if INRT is omitted, particularly in the favorable group. 48 However, this study does show that excellent outcomes can be obtained with omission of RT in patients with a negative iPET scan. This study provides a cautionary lesson though, in that the increase in relapse rate associated with omission of RT was more substantial (12%) for favorable versus unfavorable early-stage patients (2.5%), and this difference was only apparent after longer (5 years) follow-up. Despite this, chemotherapy alone is considered a reasonable treatment option, especially for patients felt to be at increased risk for late toxicities of RT or for patients who wish to avoid the risks of RT, with over 99% of patients alive at 5 years.
Similar results were shown in the RAPID trial, in which patients with limited-stage cHL underwent 3 cycles of ABVD followed by PET assessment. 67 Patients with a negative PET (n = 426) were then randomized to RT (n = 209) versus no further therapy (n = 211). At a median of 60 months of follow-up, 3-year PFS was 94.6% in the RT group and 90.8% in the chemotherapy alone group. Similar to the H10 trial, it was concluded that chemotherapy alone was statistically inferior to CMT in terms of PFS. However, also similar to the H10 trial, the RAPID trial demonstrated that excellent results can be obtained in early-stage cHL patients with omission of RT, if iPET scan is negative after 3 cycles of ABVD, as there was no survival difference. These findings indicate that, when relapses occur as a result of omission of RT, such patients can be effectively treated later.
In the ongoing GHSG HD16 trial, patients with early-stage favorable cHL will be randomly assigned to a standard approach (ABVD × 2 cycles followed by 20-Gy IFRT) versus an experimental approach in which they receive ABVD for 2 cycles and then undergo PET scan. If the PET remains positive, they will receive 20-Gy IFRT. If the PET is negative, they will receive no further therapy. This trial could ultimately define ABVD for 2 cycles as a treatment option.
It is clear from these studies that omission of RT results in a somewhat higher rate of relapse but can be considered in selected patients. However, taking a less aggressive frontline approach may also be justified by the fact that, for those who do relapse, successful salvage therapies are available. Aggressive salvage therapy with autologous stem cell transplantation historically can cure approximately 50% of relapsed patients. With new and emerging therapies for relapsed disease, such as brentuximab vedotin and the PD-1 inhibitors (eg, nivolumab and pembrolizumab), the ability to cure relapsed patients may improve even more, further calling into question the practice of applying CMT uniformly for early-stage patients undergoing first-line therapy. Unfortunately, there is insufficient data from large randomized studies with long-term follow-up to fully address this issue currently, and there remains some controversy around this issue. NCCN recommends restaging PET/CT after 3 cycles of ABVD if a chemotherapy alone treatment modality is chosen. If the Deauville score is 1 or 2, either observation or 1 additional cycle of ABVD is recommended. 46
Early-Stage Unfavorable cHL
In the United States, historically early-stage unfavorable Hodgkin lymphoma has been treated with CMT, most commonly 4 to 6 cycles of ABVD followed by consolidative RT. With this approach one can expect a 5-year PFS of approximately 80% to 85%. 58,64,86 The GHSG HD8 trial showed that RT volume size reduction from extended-field to involved-field after COPP + ABVD chemotherapy for 2 cycles produced similar results and less toxicity in patients with early-stage unfavorable cHL. 86 The GHSG trial HD11 established ABVD for 4 cycles plus 30-Gy IFRT as a standard for early unfavorable Hodgkin lymphoma. The freedom from treatment failure at 5 years was 85.0%, and overall survival was 94.5%. 68
In the HD14 study by the GHSG, patients with early unfavorable cHL were treated with 2 cycles of escalated BEACOPP followed by 2 cycles of ABVD, versus 4 cycles of ABVD. All patients then received 30 Gy of consolidative IFRT. A 5-year PFS of 95% was seen in the experimental arm, compared with 89% in the standard (ABVD) arm. As expected, this regimen was associated with more acute hematologic toxicity, and there was no difference between the 2 regimens with respect to overall survival or fertility. 69 Given the lack of improved survival and increased toxicity, ABVD has remained the standard chemotherapy regimen for early unfavorable cHL in the United States. NCCN recommends a restaging PET scan after 2 cycles of ABVD and to continue with 2 to 4 cycles of ABVD or escalated BEACOPP with or without ISRT based on Deauville scores.
Another viable treatment option is the Stanford V regimen, a condensed, 12-week regimen that includes mechlorethamine, doxorubicin, vinblastine, prednisone, vincristine, etoposide, and bleomycin, followed by IFRT. 87 In a randomized phase 3 trial conducted by ECOG (E2496), patients with stage I/II Hodgkin lymphoma with bulky mediastinal disease or advanced-stage disease were randomized to ABVD × 6 to 8 cycles versus Stanford V. RT was given (36 Gy) for those with bulky mediastinal disease or to sites of disease greater than 5 cm in the Stanford V arm. In a subset analysis focusing only on those with stage I/II bulky mediastinal disease, the 5-year failure free survival was 85% versus 79% and the 5-year overall survival was 96% versus 92% for the ABVD versus Stanford V arms, respectively. These differences were not statistically significant. 70 While the Stanford V regimen has the advantages of a 12-week treatment duration and a lower cumulative amount of bleomycin and doxorubicin, the Stanford V arm had higher rates of grade 3 lymphopenia and grade 3 to 4 peripheral neuropathies. In addition, Stanford V requires that most patients undergo RT (to original sites of disease measuring 5 cm or more plus contiguous areas). As a result, the investigators concluded that ABVD × 4 cycles plus IFRT remains the standard of care for patients with early unfavorable Hodgkin lymphoma with bulky mediastinal disease.
Other regimens have been studied in hopes of reducing toxicity, including the EVE regimen (epirubicin, vinblastine, and etoposide). This regimen was compared to ABVD in early unfavorable Hodgkin lymphoma patients, with all patients undergoing the same RT program. No differences were observed between the ABVD and EVE arms in terms of complete remission rate and overall survival. However, patients who received EVE had a significantly worse outcome than those who received ABVD in terms of relapse-free survival and failure-free survival. 88 EBVP (epirubicin, bleomycin, vinblastine, and prednisone) followed by IFRT was less efficacious compared with MOPP/ABV–type therapy. 58
An area of active investigation is whether RT can be safely omitted in patients with early- stage unfavorable cHL. The EORTC H10 study showed that, for patients with a negative iPET scan (after 2 cycles), the 5-year PFS rates were 92.1% versus 89.6% for ABVD plus INRT versus ABVD alone, respectively. While this technically did not meet criteria for noninferiority of ABVD alone, this study demonstrated that, for those with negative iPET, ABVD × 6 cycles (without radiation) can result in long-term remission in a high proportion (89%) of patients. For iPET-positive patients, 2 cycles of escalated BEACOPP were given followed by 30 Gy of IFRT on the experimental arm. This resulted in a 5-year PFS of 90.6% versus 77.4%, suggesting this may be a preferred approach for early-stage unfavorable patients with a positive iPET. 48 Even though the noninferiority of ABVD alone could not be established based on the statistical design of the study, the current NCCN guidelines recommend restaging after 2 cycles of ABVD for stage I or II unfavorable cHL and using that iPET as a guide, based on Deauville scores. For scores 1–3, ABVD × 2 cycles (total 4 cycles) plus ISRT or AVD × 4 (total 6) with or without ISRT is recommended. For a Deauville score of 4, escalated BEACOPP × 2 cycles or ABVD × 2 cycles (total 4) followed by ISRT is recommended. If the Deauville score is 5, further treatment decisions should be made based on repeat biopsy results. A follow up PET/CT is recommended for Deauville scores of 4 and 5 to confirm complete response. 46
LATE EFFECTS AND THE EVOLUTION OF RADIATION THERAPY
The RT given in Hodgkin lymphoma has evolved considerably over the years, from extended field or subtotal nodal fields developed in the 1960s, to the more focused involved-field or even involved-site radiation commonly given now. This approach reduces radiation volumes, and it already is becoming evident that the relative risk of breast cancer among young females receiving mediastinal RT for Hodgkin lymphoma is declining. 89 Cardiac dose is reduced significantly with IFRT compared to older radiation techniques as well. The extent of radiation may be reduced even further with involved-nodal/involved site or intensity-modulated approaches. 90
With new RT techniques allowing for more focused therapy and lower doses of radiation, models predict that the rate of long-term complications will decline further. 91,92 Furthermore, response-adapted (ie, PET-directed) approaches, as discussed in detail earlier in the article, are expected to increasingly allow for identification of patients who can safely avoid radiation entirely, which will hopefully lead to an even lower rate of late complications of therapy.
MONITORING FOR RELAPSE
A number of recent studies have shown that, for patients who achieve complete remission with first-line therapy, performing repeated scheduled surveillance imaging does not improve outcomes. In fact, most relapses are detected by the patient (due to symptoms or recurrence of lymph node enlargement). It is rare that a relapse would be detected by surveillance imaging alone. Furthermore, surveillance that includes routine imaging has not been associated with improved survival. 93 As a result, it is now recommended that patients undergo regular follow-up with symptom review, physical exam, and basic laboratory studies. Imaging studies should be obtained as needed for patients who develop signs, symptoms, exam findings, or laboratory values concerning for relapse.
More important than scheduled surveillance imaging for relapse is monitoring for late effects of therapy. These fall into several broad categories such as cardiovascular disease (coronary disease, congestive heart failure, valvular disease, carotid artery disease), pulmonary disease, hypothyroidism, and secondary malignancies. Aggressive surveillance for breast cancer is especially warranted in female patients who underwent chest radiation. 46
CONCLUSION
Hodgkin lymphoma is characterized pathologically by the presence of HRS cells accompanied by a polymorphous cellular infiltrate. It is a disease with a bimodal age distribution, several pathologic subtypes, and numerous treatment options. Overall, the prognosis for patients with early-stage disease is excellent, and although a majority of patients can now be cured, further studies are needed to optimize treatment such that short- and long-term treatment-related toxicities are minimized, without compromising disease control and cure.