Another approach studied in older patients is the use of R-CHOP with rituximab maintenance. In a large European study, 560 patients 60 years of age or older with advanced-stage MCL were randomly assigned to either R-FC (rituximab, fludarabine, and cyclophosphamide) every 28 days for 6 cycles, or R-CHOP every 21 days for 8 cycles. Patients who had a response then underwent a second randomization, with one group receiving rituximab maintenance therapy. Maintenance was continued until progression of disease. Patients in this study were not eligible for high-dose chemotherapy and autoSCT. The study found that rates of CR were similar with both R-FC and R-CHOP (40% and 34%, respectively; P = 0.10). However, the R-FC arm underperformed in several arenas. Disease progression occurred more frequently with R-FC (14% versus 5% with R-CHOP), and OS was shorter (4-year OS, 47% versus 62%; P = 0.005, respectively). More patients also died in the R-FC group, and there was greater hematologic toxicity compared to R-CHOP. At 4 years, 58% of the patients receiving rituximab remained in remission. Among patients who responded to R-CHOP, rituximab maintenance led to a benefit in OS, reducing the risk of progression or death by 45%.26 At this time, studies are ongoing to establish the benefit of rituximab maintenance after BR.
Bendamustine in combination with other agents has also been studied in the frontline setting. Visco and colleagues evaluated the combination of bendamustine with rituximab and cytarabine (R-BAC) in older patients with MCL (age 65 or older).63 This phase 2, two-stage study enrolled 40 patients and had a dose-finding arm for cytarabine in combination with BR. It permitted relapsed/refractory patients, but 50% had newly diagnosed, previously untreated MCL. The regimen had an impressive ORR of 100%, with CR rates of 95% for previously untreated patients. PFS at 2 years was 95%. R-BAC was well tolerated, with the primary toxicity being reversible myelosuppression.
BR was combined with the proteasome inhibitor bortezomib and dexamethasone in a phase 2 study.64 This Lymphoma Study Association (LYSA) study evaluated 76 patients with newly diagnosed MCL older than age 65 years. BR was administered in standard doses (bendamustine 90 mg/m2 on days 1 and 2 and rituximab 375 mg/m² IV on day 1) and bortezomib was administered subcutaneously on days 1, 4, 8, and 11, with acyclovir for viral prophylaxis. Patients received 6 cycles. The ORR was 87% and the CR was 60%. Patients experienced toxicity, and not all bortezomib doses were administered due to neurotoxic or hematologic side effects.
A randomized phase 3 study compared R-CHOP to the VR-CAP regimen (R-CHOP regimen but bortezomib replaces vincristine on days 1, 4, 8, 11, at 1.3 mg/m2) in 487 newly diagnosed MCL patients.27 Median PFS was superior in the VR-CAP group compared with R-CHOP (14.4 months versus 24.7 months, respectively). Additionally, rates of CR were superior in the VR-CAP group (53% compared to 42% with R-CHOP). However, there was more hematologic toxicity with VR-CAP. On the basis of these findings, the U.S. Food and Drug Administration approved bortezomib for the frontline treatment of MCL.
Other chemoimmunotherapy combinations containing bortezomib have been studied in frontline MCL treatment, with promising results. These include bortezomib in combination with R-CHOP or modified R-hyper-CVAD, as well as bortezomib in combination with CHOP-like treatments and purine analogues.27,30–32 The ongoing ECOG 1411 study is currently evaluating bortezomib added to BR for induction therapy of newly diagnosed MCL in a 4-arm randomized trial. Patients receive BR with or without bortezomib during induction and are then randomly assigned to maintenance with either rituximab alone or rituximab with lenalidomide. Other novel combination agents are actively being studied in frontline MCL treatment, including lenalidomide and rituximab and BR with lenalidomide.
Intensification of Therapy and AutoSCT: Fitter and/or Younger Patients
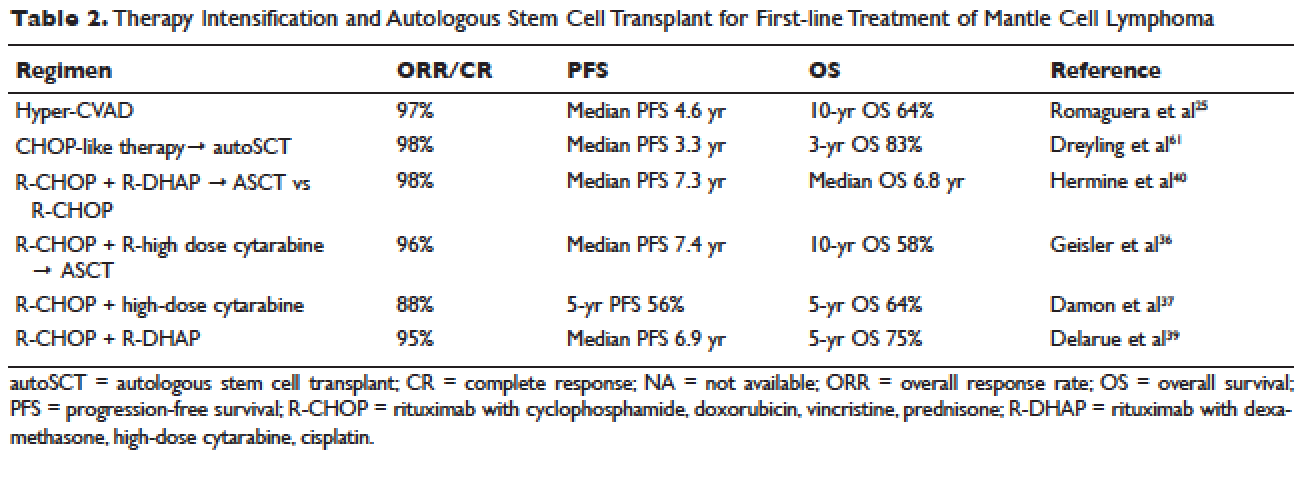
Short response duration has created the need for post-remission therapy in MCL. One approach to improve remission duration in MCL is to intensify induction through the use of cytarabine-containing regimens and/or consolidation with high-dose chemotherapy, typically using BEAM (carmustine, etoposide, cytarabine, melphalan) and autoSCT (Table 2). The cytarabine-containing R-hyper-CVAD regimen, developed at the MD Anderson Cancer Center, resulted in a 97% ORR and an 87% CR rate, with TTF of nearly 5 years. However, nearly one third of patients were unable to complete treatment due to toxicity, and 5 patients developed secondary myelodysplastic syndrome or acute myeloid leukemia.33 The feasibility of this R-hyper-CVAD regimen was tested in a multicenter cooperative group setting, but similar results were not seen; in this study, nearly 40% of patients were unable to complete the full scheduled course of treatment due to toxicity.34