Colon cancer is not common in childhood even though cases have been reported in children and adolescents.1,2 Although it is sporadic, it can arise in the setting of predisposing illnesses such as familial polyposis syndrome or inflammatory bowel disease.2-5 Only 1 or 2 cases per million children are reported globally each year, but the incidence has been noted to be on the rise.2 The nonspecific gastrointestinal symptoms and anemia as features of the disease could also be seen in other common childhood ailments, such as helminthiasis in our region in West Africa. As a result, unless there is a high index of suspicion at the outset, there is a risk that colon cancer will be diagnosed at a late stage, especially in children with no apparent predisposing factor.
In this case, an 11-year-old girl presented to our institution with abdominal pain, melena, abdominal swelling, and iron deficiency anemia. A positive family history of colon cancer in the mother and a brain tumor in an elder sibling prompted a search for and subsequent diagnosis of colon cancer. Her case highlights the importance of a high index of suspicion in making an early diagnosis to achieve the best possible outcomes. This case is being reported in line with the SCARE guidelines.6
Case summary and presentation
An 11-year-old girl presented to our facilty with recurrent abdominal pain of 8 months duration, a 4-month history of progressive paleness of the palms, and a month-long fever. There was an associated change in bowel habit to about 2-3 times per day, weight loss despite a preserved appetite, and black, tarry stools. A month before she presented, she developed low-grade pyrexia, dysuria, and pica. She was treated for iron deficiency anemia at a peripheral hospital where she first sought for care with oral iron, folic acid, and vitamin C, but with no improvement in symptoms.
She was the youngest of 8 children born to parents who were first cousins. Her father had died in a car accident when she was a year old, and her mother had died 6 years later after being diagnosed with and treated for colon cancer. An elder sibling died of a brain tumor at the age of 9 years.
On admission to our institution, the girl looked acutely ill. She was severely pale, but afebrile and anicteric. She had no petechial or purpuric skin rashes, but had glossitis with areas of papules on the anterior two-thirds of the dorsum of the tongue. She had no gingival hypertrophy, but had significant peripheral lymphadenopathy and weighed 67% of the weight for her age. In addition, she had generalized abdominal pain and a soft, well-circumscribed tender mass located at the right iliac fossa was palpated and estimated to be 8 cm x 6 cm.
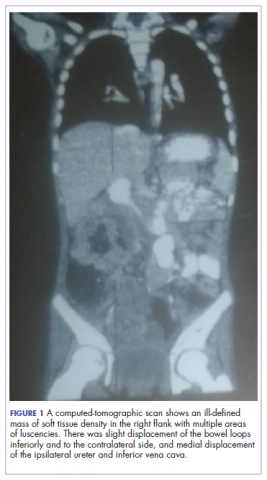
A full blood count showed severe hypochromic microcytic anemia, with a red blood cell count of 2.53 x 1012/L, packed cell volume of 9%, white blood cell count 9.4 x109/L, platelet cell count of 453 x 109/L, mean corpuscular volume of 48.6 fl, and a red cell distribution width of 23.7%. Iron studies could not be done because we lacked the facilities, but a bone marrow aspiration biopsy showed reduced bone marrow iron stores. A fecal occult blood test was positive for blood, but negative for culture, ova, or cysts. An abdominopelvic ultrasound showed the well-circumscribed mass at the right iliac fossa, and that was confirmed by a computed-tomographic scan (Figure 1).
An upper endoscopy revealed fundal and prepyloric erosions and reflux eosophagitis. Although findings from a sigmoidoscopy were normal, a histology of biopsied tissues showed features of chronic inflammation.
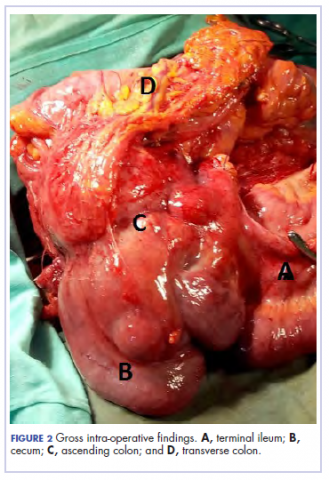
There was a delay in arriving at the final diagnosis because the patient’s family faced financial difficulties and some of the imaging procedures were not available at our institution. Other diagnoses that were entertained and managed in this case were iron deficiency anemia from peptic ulcer disease. Six weeks after her initial presentation to our institution, the patient had an exploratory laparotomy. The findings intra-operatively were those of a huge tumor involving the ascending colon measuring 16 x14 cm and extending to involve the cecum and mesenteric lymph nodes (Figure 2).
Kidneys, liver and spleen were macroscopically normal. An assessment of Duke’s stage 3C colon cancer was made and she had an extended radical hemicolectomy with anastomosis.
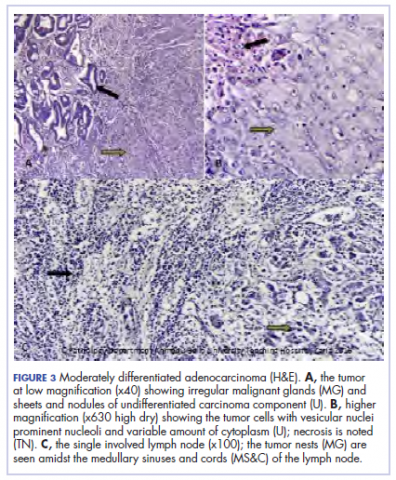
A 44.5-cm long right hemicolectomy segment comprising a 17-cm ileal segment, a 6-cm cecum, 21.5-cm ascending colon, and an 8-cm appendix was removed. The tumor was located in the ascending colon at 7.5 cm from the distal resection margin and extending 1 cm into the cecum. It had a circumference of 27 cm with fibrinous exudates on its peritoneal surface. Dissection revealed uneven circumferential thickening of the bowel wall, luminal dilatation, marked mucosal ulcerations, and liquid content made up of fecal material and necrotic debris. The tumor cut surface was solid white. We also removed 4 lymph nodes. Other uninvolved areas showed focal mucosal hyperemia, but no polyps were observed. Histology showed moderately differentiated adenocarcinoma (pT4) with ¼ nodal involvement (Figure 3).
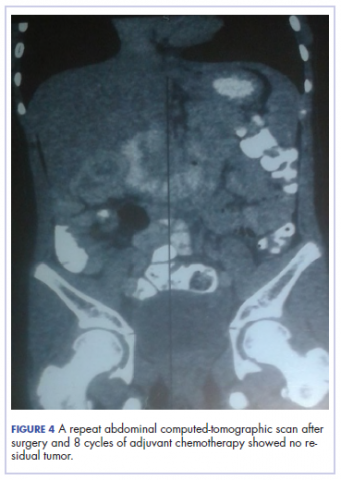
The patient’s postoperative course was uneventful, and she had adjuvant chemotherapy with oral capecitabine and intravenous oxaliplatin. She completed the 8-cycle protocol with excellent clinical response and minimal adverse events were recorded. A repeat abdominal CT scan showed no residual tumor (Figure 4), and her full blood count showed normal hematological profile with no evidence of iron deficiency.
She is presently on follow up 2 years after confirmation of the diagnosis. (Her histological diagnosis was made June 2016, and her last clinic follow-up was March 2018.
Discussion
Our patient presented with symptoms of abdominal pain, dysuria, melena, and pallor as in other case reports.7-10 A diagnosis of iron deficiency anemia was initially entertained in view of the hematologic profile, and for which management was instituted. The findings of gastric and duodenal erosions on endoscopy further supported the assumption for and treatment of peptic ulcer disease. Iron deficiency in this patient was owing to chronic blood loss from a tumour located at the upper parts of the. Vague and nonspecific symptoms are associated with delayed diagnosis and poor prognosis.1-5,11 Nonspecificity of symptoms is typical feature of colon cancer as reported in other studies.1,11-13 However, the strong family history of colon cancer heightened suspicion in this case, otherwise the diagnosis of an ascending colon tumor could have been delayed until much later and with graver consequences.
The diagnosis of colon cancer in this child was made about a year after her initial symptoms, and 3 months after her presentation to us. Ascending and transverse colon cancers are usually diagnosed late because the symptoms of intestinal obstruction – frank bleeding – will not present until the illness is substantially advanced. Ameh and Nmadu reported a case series of 8 patients from our facility with rectosigmoid tumor, of whom 6 had mucinous adenocarcinaoma and 5 of those 6 had stage 3C disease. Although the patient in the present case had an advanced disease at diagnosis, she had a moderately differentiated histology in contrast to the 6 previously reported cases, who had mucinous histology.14
Previous studies have shown that colorectal carcinoma is a rare disease worldwide, with an annual age-adjusted incidence of 0.38 people/million.1,2 When it occurs in the young, familial or hereditary predisposition should be highly suspected.1-3 To date, there is scant literature on children younger than 16 years in Nigeria.15 Various studies have found a relationship between patients with early-stage colon cancer and inherited genetic predisposition to the disease.2,5 Familial adenomatous polyposis syndrome is an autosomal dominant disorder characterized by the development of polyps during the first decade of life, extensive polyposis in the second decade, and transformation into frank carcinoma in early adulthood.1-5
Although our patient’s mother was diagnosed with and died of colon cancer, the type of which could not be ascertained because her records could not be traced. However, the operative and histological findings in this patient did not suggest the presence of polyposis. The clinical phenotype for the autosomal recessive mismatch repair deficiency includes susceptibity to glioma, leukemia, lymphoma, and colorectal carcinoma in children and young adults.1,5 Screening for genetic markers in the child in the present case might have identified the genetic abnormalities involved and would have been invaluable in the evaluation of her 6 surviving siblings and further management of this family. In conclusion. A high index of suspicion should prompt inclusion of colon cancer in the differential diagnosis of nonspecific gastrointestinal symptoms associated with colon cancer in children.
Acknowledgment
The authors obtained written informed consent from the patient and her elder sibling before writing this report. In addition, the authors thank all the staff involved in the management of this child in the pediatric medical and surgical wards.