Irinotecan-based regimens have also been extensively studied in the first-line treatment of metastatic gastric cancer, particularly as an alternative to platinum-based therapy, but superiority has not been established. The combination of fluorouracil, leucovorin, and irinotecan (FOLFIRI) was compared to ECX in a phase 3 trial.22 The study enrolled 416 patients with locally advanced or metastatic gastric or GEJ cancer. At a median follow up of 31 months, the time to progression was longer in the FOLFIRI arm as compared to the ECX arm (5.1 months versus 4.2 months, P = 0.008), but there was no difference in OS (9.5 months versus 9.7 months, P = 0.95), median PFS (5.3 months versus 5.8 months, P = 0.96), or response rate (39.2% versus 37.8%). However, the FOLFIRI regimen had an improved toxicity profile, with a lower overall rate of grade 3 or 4 toxicity (69% versus 84%, P < 0.001). Given these findings, the FOLFIRI regimen is an acceptable alternative to platinum-based therapy in suitable patients.22
HER2-POSITIVE DISEASE
The HER2 proto-oncogene, initially described in breast cancer, has been implicated in several malignancies, including gastric and esophageal cancer. Overexpression or amplification of HER2 can be found in up to 30% of gastric cancers.23 For these patients, adding trastuzumab to a standard regimen of platinum and fluoropyrimidine is the standard of care. The prospective phase 3 Trastuzumab for Gastric Cancer (ToGA) trial randomly assigned 594 patients with HER2-positive gastric cancer to receive either cisplatin and fluorouracil or capecitabine and cisplatin with trastuzumab (n = 294) or without (n = 290) trastuzumab every 3 weeks for a total of 6 cycles, followed by maintenance trastuzumab until disease progression was noted.24 HER2 positivity was defined as HER2 protein overexpression by IHC (cutoff of 3+) or gene amplification by fluorescence in situ hybridization (FISH); tumors with IHC 2+ patterns were followed with FISH studies to confirm positivity. The study found a higher incidence of HER2-positive tumors in patients with GEJ tumors compared to patients with distal gastric cancers (33% versus 20%).24 In this trial, the addition of trastuzumab was associated with an improvement in OS: 13.5 months in the trastuzumab cohort versus 11.1 months in those receiving chemotherapy alone (HR 0.74, P = 0.0048). There was not a significant difference in toxicities between the 2 cohorts, with nausea, emesis, and neutropenia being the most common adverse events. Rates of overall grade 3 or 4 events were similar as well (68% in each cohort). Further exploratory analysis was also conducted according to HER2 status by dividing patients into a “high-expressor” group (n = 446), defined as patients with IHC 3+ tumors or IHC 2+ and FISH positivity, and a “low-expressor” group (n = 131), which included patients with IHC 0 or 1+ tumors. Analysis of patients in the 2 subgroups demonstrated an improved OS with the addition of trastuzumab for the high-expressor cohort, with a median OS of 16 months (HR 0.65 [95% CI 0.51 to 0.83]) compared to 11.8 months in those receiving only chemotherapy.
Dual HER2 blockade has been investigated in metastatic gastric cancer. The phase 3 randomized JACOB trial assigned 780 patients to receive either trastuzumab with a cisplatin/fluoropyrimidine regimen with or without the addition of pertuzumab; the primary end point was OS.25 A non-statistically significant trend towards improvement in OS was found in the pertuzumab arm (17.5 months) as compared with the standard of care arm (14.2 months, HR 0.84, P = 0.0565). The pertuzumab/trastuzumab/chemotherapy cohort experienced a higher incidence of diarrhea (61.6% versus 35.1% in control arm). Cardiac toxicity was comparable in the 2 cohorts.
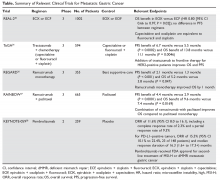
The Table provides a summary of relevant clinical trials in metastatic gastric cancer.
SECOND-LINE THERAPY
CASE CONTINUED
The patient receives capecitabine, oxaliplatin, and trastuzumab therapy for 6 cycles, followed by trastuzumab for another 3 cycles. While on therapy, he develops a painful right clavicular lesion. He undergoes magnetic resonance imaging of the right clavicle, which shows a lesion in the distal two-thirds of the right clavicle measuring 9.7 × 3.7 × 3.8 cm. The patient is started on palliative radiation to the clavicle. However, repeat CT imaging shows progressive liver metastases.