Update as of June 21: The US Food and Drug Administration (FDA) has placed the Investigational New Drug (IND) application for vadastuximab talirine on hold. No clinical trial may resume under the IND until the FDA lifts the clinical hold.
On the advice of the Independent Data Monitoring Committee, Seattle Genetics is discontinuing the phase 3 CASCADE clinical trial of vadastuximab talirine as frontline treatment in older patients with acute myeloid leukemia (AML).
The company is also suspending patient enrollment and treatment in all its vadastuximab trials, including the ongoing phase 1/2 trial in frontline high-risk myelodysplastic syndromes (MDS).
In December last year, the US Food and Drug Administration (FDA) had placed the trials of vadastuximab on full and partial clinical holds due to the potential risk of hepatotoxicity.
The FDA lifted the hold in March of this year. However, concerns regarding a higher rate of deaths, including fatal infections but not liver toxicity, in the vadastuximab arm compared to control prompted the company to discontinue the phase 3 trial.
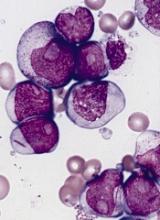
Vadastuximab talirene is an antibody-drug conjugate (ADC) targeted to CD33, which is expressed on most AML and MDS blasts. The ADC technology links anti-cancer compounds with targeting antibodies to precisely kill cancer cells and spare healthy ones.
Seattle Genetics’ ADC for Hodgkin lymphoma, brentuximab vedotin, was granted accelerated approval by the FDA in 2011.
The CASCADE trial was evaluating vadastuximab in combination with the hypomethylating agents (HMAs) azacytidine or decitabine compared to an HMA alone in older patients with newly diagnosed AML.
In addition to the MDS trial, the company is stopping enrollment onto the trial of vadastuximab in combination with 7+3 chemotherapy in newly diagnosed, younger AML patients and vadastuximab given prior to or after allogeneic hematopoietic stem cell transplant in AML patients.
Calling the decision “disappointing and unexpected,” Clay Siegall, PhD, president and CEO of Seattle Genetics, said, “Patient safety is our highest priority, and we will closely review the data and evaluate next steps.”