A novel device can significantly reduce contamination of blood cultures, according to research published in Clinical Infectious Diseases.
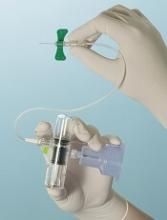
The SteriPath initial specimen diversion device (ISDD) is a blood collection system that diverts and sequesters the first 1.5 mL to 2 mL of blood drawn, which often carries contaminating skin cells and microbes.
By allowing for the disposal of this blood, the ISDD reduced blood culture contamination by 88%, when compared to standard phlebotomy procedures.
In reducing contamination, the SteriPath ISDD could reduce the unnecessary use of antibiotics, according to researchers.
“A lot of people think this is a minor problem,” said study author Mark Rupp, MD, of the University of Nebraska Medical Center in Omaha.
“However, contaminated blood cultures are a big deal. Physicians can be led astray, and patients may be harmed by additional tests and unnecessary antimicrobial therapy.”
For this study, Dr Rupp and his colleagues compared the ISDD and standard blood draw procedures, collecting a total of 1808 blood samples from 904 patients.
The researchers observed a significantly lower rate of blood culture contamination with the ISDD than with standard procedures—0.22% (2/904) and 1.78% (16/904), respectively (P=0.001).
“The 1.78% baseline rate of contamination may seem small, but we should strive to decrease adverse events to the lowest possible level because of the impact to the patient and the burden to our healthcare system,” Dr Rupp said. “We quite clearly showed the rate of contamination was significantly reduced, and that decrease has a very big impact.”
Dr Rupp and his colleagues also noted that the ISDD was about as sensitive as standard procedures. The ISDD detected true bacteremia in 7.2% (65/904) of patients, and standard procedures detected true bacteremia in 7.6% (69/904) of patients (P=0.41).
“What is important about this device is it can greatly limit the blood culture from being contaminated, so physicians are rarely fooled by false-positive results,” Dr Rupp said. “It gives clinicians confidence that results are accurate.”
This research was supported by Magnolia Medical Technologies, Inc., the company marketing the SteriPath ISDD.