infant’s stomach
The US Food and Drug Administration has approved oral propranolol hydrochloride (Hemangeol) to treat proliferating infantile hemangiomas that require systemic therapy.
The drug, which is also under review in the European Union, will be available in the US in June.
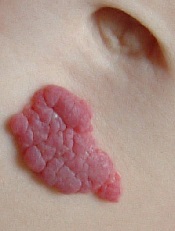
Infantile hemangioma is the most common vascular benign tumor of infancy, affecting 3% to 10% of newborns. The lesions are rarely detectable at birth and start growing noticeably in the first 4 to 6 weeks of life.
While most infantile hemangiomas do not require treatment, approximately 12% do. Depending upon their location, infantile hemangiomas might impair breathing, eating, or vision, or become life-threatening.
Propranolol has long been used in cardiology, but its use in infantile hemangiomas is relatively new. In 2007, Christine Léauté-Labreze, MD, a dermatologist at the Bordeaux University Hospital in France, discovered that propranolol could treat infantile hemangiomas.
Since then, the drug has been used for this indication off-label. And in 2009, Pierre Fabre Dermatologie began developing propranolol hydrochloride for use in infantile hemangiomas.
In a study of 32 children, propranolol slowed the growth of infantile hemangiomas in 100% of patients. Patients had received propranolol at 2 to 3 mg/kg per day for a median of 6.1 months. Side effects were “limited and mild,” according to researchers (V Sans et al. Pediatrics 2009).
Researchers also conducted a randomized, controlled trial of the drug in infants 5 weeks to 5 months old at therapy initiation. The team compared 4 propranolol treatment protocols (1 or 3 mg/kg/day for 3 or 6 months) to placebo.
Propranolol at a daily dose of 3 mg/kg for 6 months had a 60.4% success rate, compared to 3.6% in the placebo group (P<0.0001). Success was defined as complete or nearly complete resolution of the target hemangioma. However, 11.4% of patients needed to be re-treated after stopping propranolol.
The drug is contraindicated in premature infants who have a corrected age of less than 5 weeks, weight less than 2 kg, known hypersensitivity to propranolol or any of its excipients, asthma or a history of bronchospasm, pheochromocytoma, blood pressure less than 50/30 mmHg, a heart rate less than 80 beats per minute, greater than first degree heart block, or decompensated heart failure.
Propranolol hydrochloride can cause serious side effects, including hypoglycemia, bradycardia, hypotension, and bronchospasm. The drug can worsen congestive heart failure and may increase the risk of stroke in children with PHACE syndrome.
The most frequently reported adverse reactions, occurring in more than 10% of infants receiving propranolol hydrochloride, were sleep disorders, diarrhea, vomiting, and aggravated respiratory tract infections, such as bronchitis and bronchiolitis associated with cough and fever. Adverse reactions led to treatment discontinuation in fewer than 2% of treated patients.

