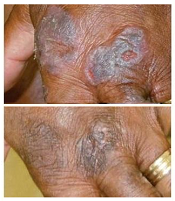
treatment (top) and 16 weeks
after treatment began
Photo from Penn Medicine
Results of a phase 1 trial suggest a topical gel can prompt regression of both treated and untreated lesions in patients with
early stage cutaneous T-cell lymphoma (CTCL).
Of the 12 patients who received the treatment, resiquimod gel, 75% had a significant improvement in treated lesions.
And 92% of patients had a more than 50% improvement in body surface area involvement, which included untreated lesions. Two patients experienced complete disease clearance.
Adverse events associated with resiquimod were largely limited to the skin, although 2 patients had transient, low-grade fever. Five patients developed superficial skin erosions that healed when treatment was stopped and did not reappear after treatment began again.
Alain Rook, MD, of the University of Pennsylvania in Philadelphia, and his colleagues reported these results in Blood.
“The results of the trial suggest that resiquimod is safely and effectively absorbed into the skin and, beyond diminishing treated lesions, also enhances the immune response, leading to healing of even untreated lesions,” Dr Rook said.
“To our knowledge, this is the first topical therapy that can clear untreated lesions and lead to complete remission in some patients.”
Treatment and response
Dr Rook and his colleagues tested resiquimod gel in 12 patients who had previously undergone an average of 6 treatments for early stage CTCL.
The patients applied specified doses of resiquimod (0.03% or 0.06%) to select skin lesions for 16 weeks. Some patients using the 0.06% dose had complete clearance of all malignant cells after 8 weeks.
By the final evaluation, treated lesions had significantly improved in 75% of patients, and 30% of patients had complete clearance of all treated lesions.
Resiquimod also improved untreated lesions, resulting in more than 50% improvement in body surface area involvement for 92% of patients.
Two participants, one of whom had been living with CTCL for more than 15 years without responding to treatment, experienced complete eradication of the disease.
Malignant cell analysis
The researchers used high-throughput sequencing to detect malignant cells in patient samples. The technique could identify a single malignant cell among 100,000 healthy cells.
The team analyzed DNA from biopsies of the same lesion before treatment and 8 weeks after treatment began.
They observed a significant reduction of malignant T cells in 9 of 10 patients tested, 3 of whom had complete eradication of the malignant population and 1 of whom had a 99.6% reduction.
Adverse events
Adverse events associated with resiquimod were all grade 1 and were primarily related to local skin irritation. There were no serious adverse events.
Five of 8 patients receiving resiquimod at the 0.06% dose developed superficial skin erosions at some sites of treatment. These erosions healed completely within a week of stopping treatment and did not recur with re-initiation of treatment.
Two patients receiving the 0.06% dose experienced 2 days of low-grade fever (less than 100.50 F) when treatment began.
“Overall, lesions responded far better to topical resiquimod than they have with other topical therapies, including some potent topical steroids and topical chemotherapy, and [resiquimod] was extremely well tolerated by patients,” Dr Rook said.
“Building upon previous research, our study suggests resiquimod might be useful in combination with other therapies in the treatment of more advanced CTCL. Further research with larger participant populations is needed to determine the best approach and application for these patients.”


