Cancer is the second leading cause of death in the United States, exceeded only by heart disease.1 Despite the overall decline in cancer death rates from 2000 through 2014, physicians struggle to accurately predict disease progression and mortality in patients with cancer who are within 6 months of death.2-8 This prognostic uncertainty makes clinical decision making difficult for patients, families, and health care providers. On a health care system level, an insight into end-of-life prognostication could also have substantial financial implications. In 2013, $74 billion was spent on cancer-related health care in the United States.9 Studies have shown that from 5% to 6% of Medicare beneficiaries with cancer consumed up to 30% of the annual Medicare payments, with a staggering 78% of costs being from acute care in the final 30 days of life.10
Rapid response teams (RRTs) were first introduced in 1995 and are now widely used at many hospitals to identify and provide critical care at the bedside of deteriorating patients outside of the intensive care unit (ICU) to prevent morbidity and mortality.11-15 Although not the original aim, RRTs are commonly activated on patients at the end of life and have therefore come to play an important role in end-of-life care.11,16 RRT activation in the oncology population is of special interest because the activation may predict higher inpatient mortality.17 In addition, RRT activation can serve as a sentinel event that fosters discussion on goals of care, change in code status, and initiation of palliative care or hospice use, particularly when also accompanied by an upgrade in level of care.11,18 As such, the ability to predict mortality after an RRT event, both inpatient and at 100 days after the event, could be of great help in deciding whether to pursue further treatments or, alternatively, palliative or hospice care.
To that end, the purpose of this study was to identify baseline patient characteristics, causes of deterioration leading to the RRT event, and vital signs and laboratory abnormalities in the peri-RRT period – the 24-hour periods preceding and following the time of the RRT event – that are associated with increased mortality, both inpatient and at 100 days after RRT activation. By choosing this acutely decompensated population, the knowledge gained may be able to guide improved advance care and end-of-life planning for terminally ill cancer patients.
Methods and materials
A retrospective study was performed at a single, 900+ bed academic center in the northeastern United States during a 2-year study period from October 2014 through November 2016. The Institutional Review Board at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania, reviewed and approved the study.
Through our institution’s RRT database, all consecutive RRT activations during the study period involving hospitalized oncology patients were reviewed. We included patients 18 years or older with a cancer diagnosis, including solid tumor and hematologic malignancy, as well as those who were status post–bone marrow transplantation (BMT), who required rapid response activation while hospitalized at our institution. We excluded patients who activated rapid response while they were in the ICU, including the BMT unit, those on the surgical floors, and those with RRT activation at other hospitals before transfer to our institution. Data for both in-hospital mortality as well as 100-day mortality for all admitted oncology patients was obtained from a separate electronic health record database at our institution from a similar time period.
Our goal was to identify patient characteristics, reasons for the RRT activation, and vital sign and laboratory abnormalities in the peri-RRT period that were associated with increased mortality, both inpatient and at 100 days after RRT activation. Our institution’s RRT database and electronic health records were accessed for data collection. Primary outcome variables for this study were inpatient and 100-day mortality post-RRT activation. We investigated the following predictor variables: age, sex, cancer diagnosis, code status at the time of RRT activation, duration from hospital admission to RRT event, length of hospital stay, time of the day the RRT event occurred (daytime vs nighttime), change in level of care (telemetry upgrade and ICU transfer), previous ICU treatment during the same hospital stay, hospice discharge, reasons cited for the RRT event (increased work of breathing, hypotension, tachyarrhythmia, change in mental status, stroke, gastrointestinal bleed, and seizure), peri-RRT lactate level, international normalized ratio (INR), hemoglobin, positive blood cultures, peri-RRT blood product administration, and scores for systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) in the 24 hours preceding the RRT activation. The SIRS includes abnormal temperature (>38°C or <36°C), heart rate of >90 bpm, increased respiratory rate of >20 times/min, and abnormal white blood cell count (>12,000 cells/mm3, <4,000/mm3, or >10% bands). Its score ranges from 0 to 4, based on the number of SIRS criteria documented. The qSOFA includes hypotension (systolic blood pressure of ≤100 mmHg), increased respiratory rate of ≥22 times/min, and altered mentation and ranges from 0 to 3 based on the number of qSOFA score documented.
Descriptive statistics were generated, and we then conducted bivariate analysis using chi-square tests or Fisher exact tests for categorical variables and simple logistic regression for continuous variables. Multivariable logistic regression models were performed to identify predictors of inpatient and 100-day mortality. Regression models were fit separately for subsets defined by the type of cancer diagnosis. Variables with P < .2 were included in the models, and backward selection method was performed, keeping variables with P < .2. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). C-statistics were used to measure goodness of fit for the models. A c-statistic value of 0.5 indicates the model is not better than random chance; a value higher than 0.7 indicates moderate accuracy, whereas a value higher than 0.8 indicates strong accuracy. P < .05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 179 hospitalized oncology patients had an RRT activation during the 2-year study period during October 2014 through November 2016. During that time, 4,654 medical oncology patients were admitted to the hospital, resulting in a rate of RRT activation of 38.4 events per 1,000 admissions. In all, 179 patients were included in the analyses for inpatient mortality, and 175 patients were included for 100-day mortality post-RRT. Patients with unknown mortality status (n = 4) at 100 days after RRT were excluded from the analyses.
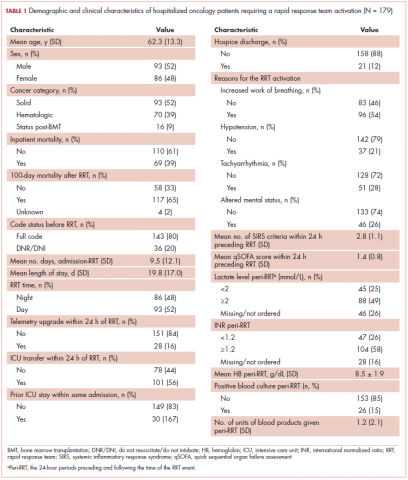
The average age of the study patients was 62.3 years (standard deviation [SD], 13.3; Table 1). They comprised equal proportions of men (52%) and women (48%). Just more than half (52%) of the patients carried a diagnosis of solid malignancy, 39% of hematologic malignancy, and 9% status post-BMT. Most of the patients were full code (80%) at the time of RRT activation. The average number of days from admission to RRT event was 9.5 days (SD, 12.1). Equal proportions of RRT events took place during the daytime (52%) and nighttime (48%), and more than half of the study patients (56%) were transferred to the ICU within 24 hours of the RRT activation. Of all the study patients, 11.7% were discharged to hospice after the RRT event, and 53% required RRT evaluation for increased work of breathing. Forty-nine percent of the total study patients had peri-RRT lactate levels ≥2 mmol/L (reference range, 0.5-2.0 mmol/L), and 58% had peri-RRT INR levels ≥1.2 (reference range, 0.85-1.15). The average SIRS score was 2.8 (SD, 1.1), and the qSOFA score was 1.4 (SD, 0.8) in the 24 hours preceding the RRT activation.
Over the 2-year study period, the inpatient mortality rate for all admitted oncology patients was 2.3% (108 deaths in 4,654 oncology inpatients), according to claims data. By comparison, of the 179 patients who required an RRT activation, 39% did not survive to discharge. When those patients were categorized based on their cancer type, 43% of the solid malignancy patients died within the same hospital stay after an RRT event, 35% of the hematologic malignancy patients died, and 25% of the status post-BMT patients died. Of the 175 patients with known mortality status at 100 days after RRT, 65% of total patients had died within that time compared with only 15.7% (347 deaths in 2,217 patients) of all admitted patients with cancer who did not experience an RRT event. When categorized based on their cancer type, significantly more patients (78%) with solid tumors had died within 100 days after RRT activation, whereas only 55% of those with a hematologic malignancy and 50% of those who were post-BMT died within the same time period.
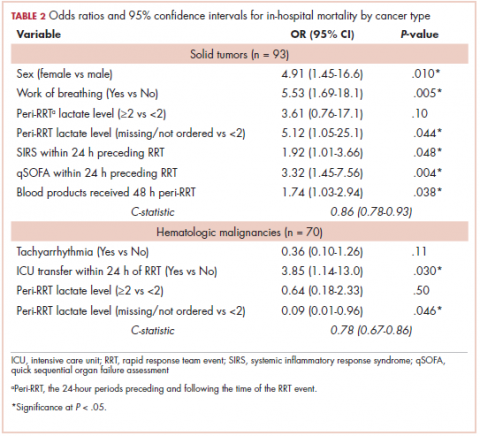
Tables 2 and 3 present major findings from regression models with a moderate to strong level of prediction. The characteristics associated with increased odds of inpatient mortality among solid tumor patients after an RRT event were female sex (OR, 4.91; 95% CI, 1.45-16.6), increased work of breathing as the reason for the RRT activation (OR, 5.53; 95% CI, 1.69-18.1), having no lactate level ordered (OR, 5.12; 95% CI, 1.05-25.1), each unit increase in SIRS score (OR, 1.92; 95% CI, 1.01-3.66), each unit increase in qSOFA score (OR, 3.32; 95% CI, 1.45-7.56), and each unit increase in peri-RRT blood products being given (OR, 1.74; 95% CI, 1.03-2.94). Among hematologic malignancy patients, ICU transfer within 24 hours of the RRT (OR, 3.85; 95% CI, 1.14-13.0) was associated with increased inpatient mortality, whereas having no lactate level ordered (OR, 0.09; 95% CI, 0.01-0.96) was associated with lower odds of inpatient mortality.
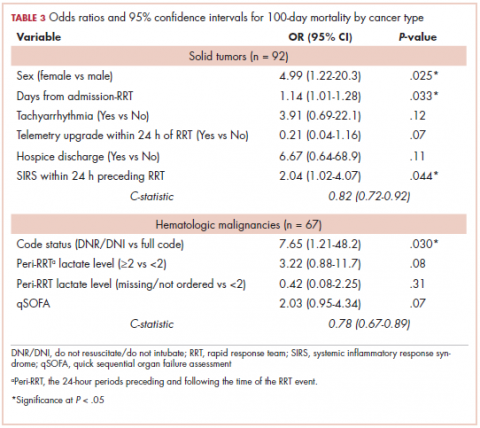
The characteristics associated with increased odds of 100-day mortality in patients with solid tumors were female sex (OR, 4.99; 95% CI, 1.22-20.3), increase in each day from admission to RRT event (OR, 1.14; 95% CI, 1.01-1.18), and each unit increase in SIRS score (OR, 2.04; 95% CI, 1.02-4.07). For hematologic malignancy patients, being do not resuscitate (DNR) or do not intubate (DNI) (OR, 7.65; 95% CI, 1.21-48.2) was associated with increased odds of 100-day mortality.
Discussion
The results of the study highlight the very high mortality rates associated with oncology patients requiring RRT activations, with 39% of patients dying within the same hospital stay and 65% dying within 100 days of the RRT event. These results are particularly notable when contrasted with the 2.3% inpatient and 15.7% 100-day postdischarge mortality rates in the total oncology patient population over a similar time period. The inpatient mortality rate after an RRT activation in our study closely resembled the rate reported by Austin and colleagues, which was 33% (hospital mortality in oncology patients cited during the time was 48.2 deaths per 1,000 patient admissions).17 Of note in our study is that solid tumor patients had higher mortality than the hematologic malignancy patients; 43% died within the same hospital stay and 78% died within 100 days, compared with 35% and 55%, respectively, in patients with hematologic malignancies. The poor prognosis of oncology patients requiring an RRT evaluation must be conveyed to the patients and families and taken into consideration by health care team to determine the most appropriate course of care subsequent to RRT activation.
Our finding that female sex is significantly and strongly associated with increased inpatient and 100-day mortality in patients with solid tumors was unexpected. The cause for this disparity remains elusive. We noted that, in our study, the following types of malignancies were more common in women than men (comparison of women vs men shown in parentheses): lung (53% vs 47%), colon (60% vs 40%), acute lymphoblastic leukemia (83% vs 17%), diffuse large B-cell lymphoma (64% vs 36%), and multiple myeloma (58% vs 42%). Whether these types of cancers are more clinically aggressive and associated with earlier mortality post-RRT could not be ascertained from our data. Gender bias in clinicians’ bedside determination of severity of illness may also play some role in this substantial mortality gap.
Among all the causes for RRT activation, increased work of breathing was the only variable associated with increased inpatient mortality in solid tumor patients. In a study by Austin and colleagues, decreased oxygen saturation was the most common reason for the RRT evaluation, though it did not reach statistical significance as a predictor of inpatient mortality.17 SIRS and qSOFA scores in the 24 hours preceding the RRT event along with peri-RRT blood product administration were all significant predictors of inpatient mortality among patients with solid tumors but were not so for those with hematologic malignancies. It is interesting to note that low hemoglobin was found to be associated with inpatient mortality in a study on 456 hospitalized patients with solid tumors (there was no data on RRT evaluation in their dataset).13 The fact that these well-validated measurements of illness severity correlate positively with RRT activation and increased mortality is intuitive and lends external credibility to other findings in this study.
In patients with hematologic malignancies, ICU transfers within 24 hours of the RRT activation were associated with 4-fold increased odds of inpatient death. This was not shown to be the case in patients with solid tumors. This should be explored in future studies because it could be crucial in conducting goals-of-care discussions in terminally ill cancer patients. The study also showed that patients with hematologic malignancies who were DNR or DNI were associated with almost 8-fold increased odds of 100-day mortality. This argues for a fair predictive ability of the care teams in this particular subgroup. Conversely, hospice referral is underused; of the patients that died at 100 days after the RRT event, only 16.2% were referred to hospice at the time of discharge.
Limitations
Limitations of the study include its retrospective nature at a single medical center on a small group of study participants. Variables such as lactate dehydrogenase level and Eastern Conference Oncology Group Performance Status, which have been found to be predictive of increased mortality in hospitalized oncology patients,19 were not consistently available for analysis in the data set. We had 4 patients whose mortality status was not known at 100 days and were excluded from the study. Because of a lack of documentation, we were also not able to reliably collect the data on patients with multiple RRT events. This presumably would be associated with increased mortality on its own. We only included the data associated with the earliest RRT activation in our electronic health records.
In addition, it is important to note that 26% and 16% of the study patients had missing lactate and INR values, respectively. Given the small size of the study and the unclear significance of the missing lactate and INR, we opted to include the patients with the missing data for final analyses of the regression models. The significance of a care team not ordering a lactate level is perhaps associated with the reason for RRT activation (ie, the patient seemed to be less ill) and perhaps could be associated with non–sepsis-related RRT events.
Conclusions
This study reports on the outcomes of oncology patients admitted to the hospital whose clinical deterioration required activation of a rapid response team. Female sex, increased qSOFA and SIRS scores in the 24 hours preceding the RRT event, and the need for blood product administrations around the time of the RRT event correlated with increased inpatient mortality. Hospitalized oncology patients’ d undestood and response evaluation if perPatientoutcomes, both regarding inpatient and 100-day mortality, demonstrated surprisingly poor survival, with solid malignancy patients bearing significantly higher burden of both inpatient mortality and mortality at 100 days after the RRT event. The findings from the study could help patients, families, and providers make informed decisions regarding advance care and end-of-life planning for terminally ill cancer patients.
The Cancer Center Support Grant 5P30CA056036-17 and the Biostatistics Shared Resource and Thomas Jefferson University Hospital’s Rapid Response Team (RRT) committee.