CHICAGO – Multiparametric magnetic resonance imaging trumped national guidelines in correctly classifying patients with prostate cancer as candidates for active surveillance in a retrospective study of 126 men.
National Comprehensive Cancer Network (NCCN) guidelines misclassified 22 of the 126 patients, compared with 12 using multiparametric magnetic resonance imaging (MP-MRI).
 Courtesy Dr. Baris Turkbey
Courtesy Dr. Baris Turkbey
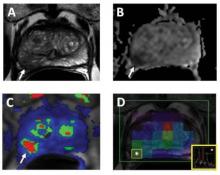
The following images represent a 62-year-old patient with a serum PSA of 3.5ng/dL. Axial T2W MR image (a) shows a hypointense lesion in the right mid peripheral zone (arrow) with slight bulge posteriorly suggesting a possible extracapsular extension; ADC map of DW MRI (b) shows restricted diffusion within the lesion (arrow); the quantitative map (c) obtained from DCE MRI demonstrates early and fast enhancement within the lesion (arrow) as well as MR spectroscopy (d) demonstrates increased choline/citrate ratio within the lesion (white asterisk). The patient underwent robot assisted radical prostatectomy and a Gleason 4 4 tumor with extracapsular extension was found in the right peripheral zone at histopathology.
When MP-MRI was added to the NCCN criteria, only 5 patients were misclassified, Dr. Baris Turkbey reported in an award-winning paper at the annual meeting of the Radiological Society of North America.
"Presently, MRI is not in any urology guideline, but we want to change this," he said. "Our future goal is to create an NCI [National Cancer Institute] prostate cancer nomogram that includes multiparametric MRI, and our scientists are close to finishing it."
Dr. Turkbey, a fellow in the division of cancer treatment and diagnosis at the National Institutes of Health in Bethesda, Md., and his colleagues evaluated 126 men with biopsy-proven prostate cancer who underwent 3T MP-MRI of the prostate and subsequent radical prostatectomy at a median of 48 days. Their mean age was 59 years and mean prostate-specific antigen (PSA) level 6.67 ng/mL.
MP-MRI images were obtained of the largest and most aggressive lesion using T2-weighted MRI, diffusion-weighted MRI, MR spectroscopy, and dynamic contrast-enhanced MRI. Each dominant lesion was then assigned an MP-MRI score of low (at least two positive sequences), moderate (three positive sequences), or high (four positive sequences).
Patients were eligible for active surveillance on MP-MRI if they had a dominant tumor of less than 0.5 cm3 without extracapsular extension or seminal vesicle invasion and a low imaging score. The NCCN criteria for active surveillance are T1c disease, Gleason score of 6 or less, fewer than three positive biopsy cores, PSA less than 10 ng/mL, and PSA density less than 0.15 ng/mL/g.
Based on histopathological findings, 14 of 126 patents were eligible for active surveillance, with the remaining 112 candidates for active whole gland treatment.
NCCN guidelines wrongly classified 5 of 14 active surveillance patients and 17 of the 112 active treatment patients, whereas MP-MRI wrongly classified 1 active surveillance and 11 active treatment patients, Dr. Turkbey said.
The sensitivity, specificity, and overall accuracy of the NCCN guidelines were 64%, 35%, and 83%, respectively (P = .00002), compared with 93%, 54%, and 91% with MP-MRI (P less than .000001).
The study was limited by using a relatively simple, nonweighted MP-MRI scoring system and comparing MP-MRI with NCCN guidelines only, he acknowledged. Dr. Turkbey said the researchers are currently evaluating a system in which the various parameters are weighted to obtain better predictions.
When asked whether MP-MRI would be cost effective in routine clinical practice, Dr. Turkbey said that "compared to the costs of doing the wrong thing to a patient, an annual or semiannual MRI is well worth it."
Dr. Turkbey reported having no conflicts of interest. A coauthor reported serving as a researcher for Koninklijke Philips Electronics, General Electric, Siemens, Hoffman-La Roche, and iCAD.

