SAN ANTONIO – Discontinuation of aromatase inhibitor therapy because of toxicity is significantly more likely to occur in breast cancer patients having a greater burden of specific symptoms even before starting on the endocrine agent.
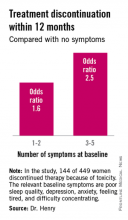
The baseline predictive symptoms identified in the randomized prospective Exemestane and Letrozole Pharmacogenetics (ELPh) trial were self-reported depression, anxiety, poor sleep quality, difficulty concentrating, and a tired feeling. Patients with three to five of these symptoms before going on an aromatase inhibitor (AI) were 2.5-fold more likely to stop treatment because of toxicity within the first 12 months than were those with none of the symptoms, Dr. Norah L. Henry reported at the San Antonio Breast Cancer Symposium.
Early discontinuation of AI therapy because of toxicity is a big problem, occurring in 20%-30% of patients who start on treatment. In an earlier study by Dr. Henry and her colleagues, discontinuation was most often from arthralgias or other musculoskeletal complaints (J. Clin. Oncol. 2012;30:936-42).
The ELPh trial included 449 evaluable postmenopausal women with early-stage estrogen receptor–positive breast cancer who were randomized to open-label exemestane or letrozole for 2 years.
To test the study hypothesis that certain baseline patient-reported symptoms increased the likelihood of early treatment discontinuation, participants were evaluated at baseline and again 1, 3, 6, 12, and 24 months after starting on an AI. At each time point, depression was self-rated using the Center for Epidemiologic Studies Depression Scale, sleep disturbance by the Pittsburgh Sleep Quality Index, and anxiety by the Hospital Anxiety and Depression Scale. Other baseline self-reported symptoms included in the prospective evaluation were joint pain, vaginal dryness, forgetfulness, difficulty concentrating, and feeling tired, explained Dr. Henry, a medical oncologist at the University of Michigan, Ann Arbor.
One hundred forty-four of the 449 women (31.2%) discontinued AI therapy because of toxicity by 12 months. In a multivariate logistic regression analysis, two of the baseline symptoms turned out to be independently associated with a significantly increased rate of treatment discontinuation: poor sleep quality as defined by a PSQI score greater than 5, reported by 45% of subjects at baseline, was associated with a 1.8-fold increased risk; and moderate or severe difficulty in concentrating was associated with a 2.6-fold increased likelihood of treatment discontinuation.
While the other symptoms under study were not individually associated with an increased risk of treatment discontinuation, the collective burden imposed by having a greater number of baseline symptoms was associated with an increased risk.
Several earlier studies by other investigators had identified prior chemotherapy, older age, and greater body mass index as being predictive of nonpersistence with AI therapy. Interestingly, neither prior chemotherapy nor greater body mass index was associated with early treatment discontinuation in the ELPh study, Dr. Henry noted.
Patients assigned to exemestane were 63% more likely to halt treatment within 12 months than were those randomized to letrozole.
The clinical relevance of these ELPh study findings is that early identification of patients with a greater burden of baseline symptoms predictive of nonadherence might improve persistence on AI therapy.
"Up-front management of these symptoms rather than waiting until symptoms become particularly problematic may improve persistence with AI therapy," she said.
Possible interventions might include preferential use of letrozole or tamoxifen in such patients, adoption of an exercise program or behavioral intervention, or pharmacologic therapy with an SSRI or a serotonin–norepinephrine reuptake inhibitors, a strategy now under study in the ongoing SWOG S1202 trial, in which patients are randomized to duloxetine or placebo.
Audience member Steven E. Vogl called the ELPh results important information.
"It recalls the history of chemotherapy-induced cognitive impairment, which in the latest couple of analyses seems to exist before the chemotherapy," observed Dr. Vogl of Bronx, N.Y.
The ELPh study was conducted by the Consortium on Breast Cancer Pharmacogenomics and funded chiefly by the Damon Runyon Cancer Research Foundation. Dr. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.