DURBAN, SOUTH AFRICA – HIV-infected women who remained on antiretroviral therapy throughout the postpartum period reduced their risk of clinical stage 2 or 3 HIV disease events by 53%, compared with those who stopped treatment postpartum in the PROMISE 1077HS trial, Judith Currier, MD, reported at the 21st International AIDS Conference.
PROMISE (Promoting Maternal and Infant Survival Everywhere) is an ongoing multinational, multicomponent series of clinical trials. PROMISE 1077HS was designed to assess the risks and benefits of continued antiretroviral therapy (ART), compared with stopping therapy among nonbreastfeeding women postpartum, explained Dr. Currier, professor of medicine and chief of infectious diseases at the University of California, Los Angeles.
PROMISE 1077HS included 1,653 HIV-positive women in the United States and seven low- or middle-income countries. All participants were relatively healthy as evidenced by their median CD4+ count of 550 cells/mm3 prior to starting ART in pregnancy. None were planning to breastfeed. Upon delivery, the women were randomized to continue ART – the chief regimen was ritonavir-boosted lopinavir (Kaletra) plus tenofovir/emtricitabine (Truvada) – or stop therapy, restarting only when their CD4+ count fell below 350 cells/mm3.
Participants were prospectively followed for a median of 2.3 years post delivery. At that point, in summer 2015, the results of the landmark START trial were released (N Engl J Med. 2015 Aug 27;373[9]:795-807), paving the way for the current global strategy of ART for life in HIV-infected patients regardless of their CD4+ cell count.
The primary efficacy endpoint in PROMISE 1077HS was a composite of an AIDS event, death, or a serious cardiovascular, renal, or hepatic event. In this relatively healthy population, too few of these events occurred to allow the researchers to draw conclusions (four in the continued-ART group and six in the controls who stopped ART post partum).
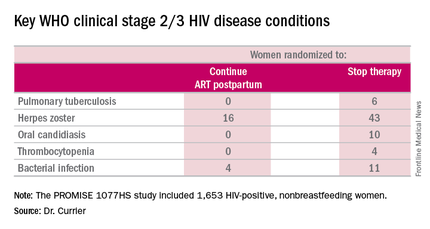
But the secondary endpoint of time to World Health Organization (WHO) clinical stage 2 or 3 HIV disease events was a different story. A total of 39 of these events occurred in the continued-ART group, for a rate of 2.02% per year, compared with 80 events and a rate of 4.36% per year in controls. That difference translated into a 53% relative risk reduction. Some key WHO clinical stage 2 and 3 HIV disease events include pulmonary tuberculosis, herpes zoster, oral candidiasis, thrombocytopenia, and bacterial infection.
Fully 23% of patients in the continued-ART group had laboratory-confirmed virologic failure as defined by a viral load of HIV RNA in excess of 1,000 copies/mL after at least 24 weeks of postpartum ART. Additionally, resistance testing indicated two-thirds of affected patients showed no evidence of resistance at the time of virologic failure, meaning their viremia was due to nonadherence to ART.
“This virologic failure rate highlights the importance of the challenge of adherence in this population,” Dr. Currier said. “Interventions to improve adherence as well as studies to examine newer regimens with a high genetic barrier to resistance are needed to ensure maximal long-term benefit from this strategy of continued ART postpartum.”
The PROMISE studies are funded by the National Institutes of Health. Dr. Currier reported having no financial disclosures.