Smokers who used e-cigarettes and other electronic nicotine delivery systems (ENDS) were less likely to quit than were those who did not use such products, according to a 2015 survey and a follow-up conducted a year later.
“Under ‘real world’ use and conditions [ENDS] may have suppressed or delayed quitting among some adult smokers,” Scott R. Weaver, PhD, and his associates at Georgia State University, Atlanta, wrote in PLoS One. The original survey, conducted in August and September of 2015, involved 1,284 U.S. adult smokers from the GfK KnowledgePanel, of whom 858 completed the follow-up survey in September 2016.
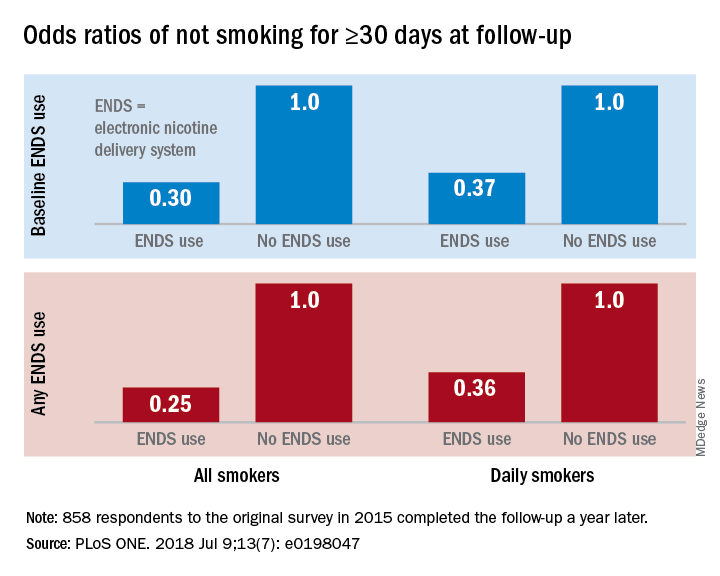
Smokers who used ENDS at baseline were slightly more likely to attempt to quit (53.7%) than were those who did not (48.6%) but were much less likely to have quit (defined as no smoking for at least 30 days at the time of follow-up): 9.4% vs. 18.9%, for an adjusted odds ratio of 0.30. Those who used ENDS at any time during the study were much more likely than were non-ENDS users to make an attempt (58.5% vs. 44.4%), but they were, again, much less likely to succeed (7.7% vs. 22.2%; AOR, 0.25), the investigators reported.
The results were similar for the subset of respondents who smoked every day: ENDS users were more likely to attempt to quit but less likely to succeed. Odds ratios for quitting were 0.37 for those using ENDS at baseline and 0.36 for those who used ENDS at any time since the first survey, Dr. Weaver and his associates said.
“Use of current ENDS products in real world conditions [does] not seem to improve the chances of quitting for smokers, and, under the current landscape, may not be the disruptive technology that increases the population quit rate and reduces the harm of combustibles,” they wrote.
The study was supported by the National Institute of Drug Abuse and the Food and Drug Administration’s Center for Tobacco Products. One of the investigators has received funding in the form of grant funding from Pfizer and the National Institutes of Health and another has served as a paid consultant to the Centers for Disease Control and Prevention.
SOURCE: Weaver SR et al. PLoS ONE. 2018 Jul 9;13(7): e0198047. doi: 10.1371/journal.pone.0198047.