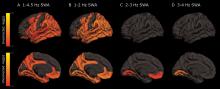
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.