NEW ORLEANS – A novel, highly attenuated, nonreplicating smallpox vaccine showed excellent safety and immunogenicity in atopic dermatitis patients in a phase II clinical trial.
"These results strongly suggest that [it] may be suitable for vaccination of atopic dermatitis patients, who are excluded from vaccination with traditional smallpox vaccines," Dr. Maria Yadira Hurley said in presenting the study findings at the annual meeting of the American Academy of Dermatology.
Called Imvamune, the vaccine is derived from the parent strain Modified Vaccinia virus Ankara (MVA-BN), a highly attenuated pox virus that has lost the capacity to replicate in human cells, according to its maker, Bavarian Nordic A/S, a Denmark-based biotechnology company.
Based upon the highly favorable results of this and more than a dozen other clinical trials totalling in excess of 2,500 subjects, the U.S. government is already stockpiling the vaccine. The government considers smallpox a top-level biologic threat and has placed high priority on developing a vaccine for individuals who aren’t supposed to receive the current vaccines, all of which are live-virus vaccines requiring viral replication in order to generate immunity, explained Dr. Hurley, a dermatologist at St. Louis University.
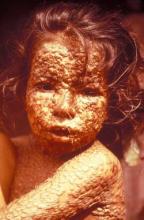
The government estimates 28 million doses of the new smallpox vaccine are needed just for patients with active atopic dermatitis or a history of the disease. Other potential recipients of the vaccine include individuals who are HIV positive or otherwise immunocompromised, as well as their household contacts.
The phase II trial in which Dr. Hurley served as principal investigator included 350 subjects aged 18-40 years with mild to moderate atopic dermatitis and 282 healthy controls, all of whom received two vaccinations 4 weeks apart.
The vaccine was well tolerated, with no cases of eczema vaccinatum, myocarditis, or pericarditis. Additionally, there was no indication that the new vaccine worsens atopic dermatitis.
The atopic dermatitis patients generated strong vaccinia-specific immune responses similar to those noted in healthy controls. Neutralizing responses were comparable to what is seen with the traditional smallpox vaccines in healthy individuals. Immune responses were greatest in both study arms 2 weeks after the second vaccination.
Smallpox is estimated to have killed 300-500 million people worldwide in the 20th century alone. Although naturally occurring variola virus has been eradicated, stored samples are known to exist in the United States and Russia, and undeclared samples may exist elsewhere. The DNA sequence of the variola virus was published in 1992, and it’s possible today to recreate the virus using gene synthesis techniques. Vaccination is the only known means of protection, Dr. Hurley noted.
She declared having received a research grant from Bavarian Nordic and being on the speakers bureaus for Abbott Laboratories and Amgen.