Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
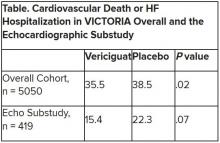
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
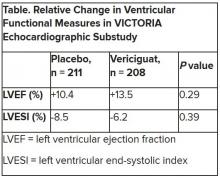
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.