The makers of bevacizumab, the cancer drug marketed as Avastin, announced Feb. 14 that they have learned that a counterfeit product has been distributed in the United States.
"The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," said makers F. Hoffmann-La Roche and Genentech in a press release.
The companies are working with the Food and Drug Administration and law enforcement to determine the source of the counterfeit drug and prevent its further distribution.
It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
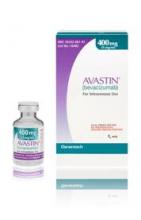
Importantly, the counterfeit product does not look like authentic Avastin.
Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (e.g., JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is English.
Health care providers who suspect that products in their possession may be counterfeit should immediately contact the FDA’s Office of Criminal Investigations (OCI) at 1-800-551-3989 or Genentech’s Product Quality Assurance Department at 1-800-334-0290.
 Courtesy of Genentech
Courtesy of Genentech
This package of counterfeit Avastin has the letter B in the lot number and a date of manufacture -- neither of which appears on packages of Avastin approved for use in the United States. Genentech is not identified on the label, and the expiry date is written entirely in numerals.
If a patient is experiencing any side effects that a health care provider thinks may be related to Avastin or that are different from those commonly associated with Avastin, the health care provider should immediately call the FDA’s MedWatch Program (1-800-FDA-1088) or Genentech’s Drug Safety Department at 1-888-835-2555.
Avastin is approved in the United States for the first- and second-line treatment of metastatic colorectal cancer in combination with intravenous 5-fluorouracil-based chemotherapy; first-line treatment of unresectable, locally advanced, recurrent or metastatic, nonsquamous, non–small cell lung cancer in combination with carboplatin and paclitaxel; treatment of metastatic renal cell carcinoma in combination with interferon-alfa; and treatment of glioblastoma, as a single agent for adult patients with progressive disease following prior therapy.