Interim results from an ongoing, registry-based phase IV surveillance study show that the medication pegvisomant is safe and effective in treating acromegaly, a disorder caused by pituitary tumors.
Among 1,288 people treated for acromegaly with pegvisomant between 2004, when the medication was authorized in Europe, and the end of 2009, serious adverse effects occurred in 159 (12.3%), and pegvisomant-related serious adverse effects in 26 (2%). No deaths were attributed to pegvisomant use, though 15 subjects (1.1% of the cohort) died while on treatment. Injection-site reactions were recorded for 28 subjects (2.2%).
 ©Elsevier Ltd. All rights reserved.
©Elsevier Ltd. All rights reserved.
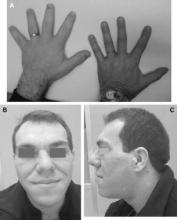
Fig. 1. As compared with the hand of a normal person (right), the hand of a patient with acromegaly (left) is enlarged, the fingers are widened, thickened and stubby, and the soft tissue is thickened (A). Facial aspect of a patient with acromegaly. The nose is widened and thickened, the cheekbones are obvious, the forehead bulges, the lips are thick and the facial lines are marked (B and C).
Importantly, pegvisomant, which works by blocking insulinlike growth factor 1 (IGF-1), was not seen to contribute to increases in tumor size. Among the 936 subjects with at least two available MRIs, 3.2% saw MRI-confirmed changes in tumor size during the study period, revealing tumor growth to be uncommon during treatment, and 63.2% of subjects had normal IGF-I levels at a mean dose of 18 mg/day after 5 years of treatment.
Adverse liver effects were rare, with elevated AST or ALT levels (higher than three times the normal upper limit) reported in 30 patients (2.5%) among the 1,178 who had at least one liver enzyme test after starting pegvisomant. However, in all subjects with elevated liver tests and follow-up laboratory results, the increases resolved. There were no reports of liver failure.
The findings, published online ahead of print (J. Clin. Endocrinol. Metab. 2012 [doi:10.1210/jc.2011-2508]), come from ACROSTUDY, a registry of people treated with pegvisomant for acromegaly for a mean of 3.7 years and followed up for an additional 2 years as of December 2009, the cutoff date for the interim analysis. About half (51%) of the subjects were male. Overall, mean age at diagnosis was 42.1 years and mean age at start of treatment was about 50 years. Most subjects (1,131) had been previously treated for their disease, 954 with surgery.
The vast majority of subjects lived in Europe, where pegvisomant is prescribed only for patients in whom other interventions have failed. This means that patients with the worst disease activity and the most comorbidities were likely overincluded, said the study’s authors, led by Dr. A. J. van der Lely of Erasmus University Medical Center in Rotterdam, the Netherlands. Most subjects (n = 1,023) had at least one reported comorbidity before starting pegvisomant, with hypertension and diabetes being the most common.
Dr. van der Lely and his colleagues noted that because the observational trial was conducted in a clinical setting, with fewer clinical visits per year than would be expected in a controlled setting, adverse effects could have been underreported.
The efficacy rate of pegvisomant was seen as lower than reported in earlier clinical trials, with only 63.2% of subjects achieving normal IGF-1 levels on treatment. Dr. van der Lely and his colleagues noted that this, too, could have been related to the administration of pegvisomant in clinical settings, where doses and compliance may have varied. They also noted that stricter criteria were used to assess IGF-1 normalization in this study than in the interventional trials, and that a different assay was used to determine IGF-1 levels. The authors concluded that, "analysis of ACROSTUDY [data] show that pegvisomant can control IGF-1 levels in a majority of patients with acromegaly who do not respond adequately to other therapies."
Dr. van der Lely and six of his coauthors acknowledged receiving ongoing honoraria from Pfizer, the manufacturer of pegvisomant. The remaining five coauthors on the study are Pfizer employees.

