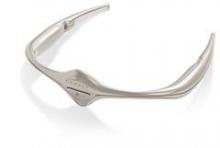
The Food and Drug Administration has authorized the marketing of Cefaly, a transcutaneous electrical nerve stimulation device that is designed for use before the onset of migraine headaches.
Cefaly would be first device of its kind in the United States, and it "provides an alternative to medication for migraine prevention," Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a statement. "This may help patients who cannot tolerate current migraine medications for preventing migraines or treating attacks."
The device, which resembles a futuristic tiara for the forehead, is battery powered and will be available via prescription for patients aged 18 years or older. It generates an electric current that stimulates branches of the trigeminal nerve, which has been associated with migraine headaches, and it should only be used once per day for 20 minutes, according to the FDA.
The device was approved based on a randomized, controlled trial of 67 patients, and a satisfaction study of 2,300 users, a little more than half of whom said they were satisfied. There were no serious adverse events in either study.
The 67-patient study showed that the mean number of migraine days, monthly migraine attacks, monthly headache days, and monthly acute antimigraine drug intake were significantly reduced among the patients who received the device, but not among those who were using the placebo device.
Meanwhile, in the 2,300-patient satisfaction study, the most common patient complaint was dislike of the feeling that the device generated. Users also reported sleepiness during the treatment session, and headache afterward.
The Cefaly kit sells for about $350 on the company’s website.
On Twitter @naseemsmiller