KEYSTONE, COLO. – A novel toll-like receptor-9 agonist showed impressive clinical efficacy in persistent allergic asthma in a double-blind, placebo-controlled clinical trial.
"This is really interesting," Dr. Harold S. Nelson commented in highlighting the European study as one of the most promising new developments in immunology during the past year in his talk at a meeting on allergy and respiratory diseases sponsored by National Jewish Health.
The toll-like receptor-9 agonist under study is known as QbG10. It consists of a protein shell derived from the Q-beta bacteriophage which is wrapped around a core of DNA oligomer G10 rich in A-type CpG motifs. The protein shell protects the G10 CpG so that when the antigen-presenting cell digests that outer coat, the CpG sits like a Trojan horse inside the T cell or macrophage. The G10 CpG induces interferon-alpha production, which in turn causes degradation of transcription factor GATA-3, explained Dr. Nelson, professor of medicine and of pediatrics at the University of Colorado, Denver, and National Jewish Health.
Allergic asthma is a disease in which allergen-specific Th2 responses figure prominently. QbG10 therapy is designed to nudge the immune system toward a Th1-mediated protective response.
What’s particularly intriguing about this novel immunotherapy, he added, is that the clinical efficacy is achieved without administering any allergen. The results are achieved simply by showing the CpG to asthma patients. CpGs are DNA oligonucleotides containing unmethylated deoxycytidylyl-deoxyguanosine dinucleotides.
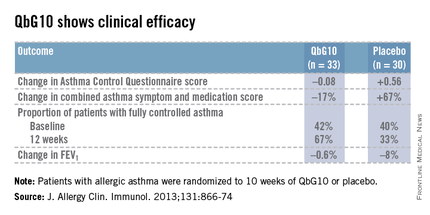
The European proof-of-concept study included 63 patients with allergic asthma being treated with moderate- or high-dose inhaled corticosteroids. They were randomized to receive seven injections of QbG10 or placebo over the course of 10 weeks, during which a controlled steroid withdrawal was carried out. After 4 weeks, the patients’ inhaled steroid dose was cut by 50%. After 8 weeks, the inhaled steroid was discontinued altogether. At evaluation at 12 weeks – 4 weeks after inhaled steroids were halted – two-thirds of QbG10-treated patients had well-controlled asthma as defined by an Asthma Control Questionnaire score of 0.75 or less, compared with one-third of controls. The QbG10 group showed similar advantages on other measures of asthma control, in spite of the steroid taper (see chart).
Local injection site reactions were common in the QbG10 group. The reactions were generally mild to moderate and were most pronounced 1-2 days post injection. The biggest reactions were noted after the third of the seven injections. No systemic reactions occurred (J. Allergy Clin. Immunol. 2013;131:866-74).
Dr. Nelson reported having no financial conflicts of interest regarding this work.