CHICAGO – The investigational "3D" combination of oral antiviral drugs achieved a cure rate that was nothing short of sensational in the phase III PEARL-II trial involving noncirrhotic patients with hepatitis C genotype 1b who had previously failed pegylated interferon–based therapy.
Moreover, these results were achieved without the use of daily oral ribavirin, formerly a standard part of hepatitis C therapy, and one responsible for a substantial burden of side effects.
Genotype 1b is the most prevalent form of hepatitis C disease worldwide. While it is especially common in Europe and Japan, genotype 1b also accounts for about one-third of cases of HCV in the United States.
The so-called 3D regimen is an all-oral combination of three direct-acting antiviral agents: ombitasvir, dasabuvir, and ABT-450. Each of these agents inhibits the viral life cycle at a different point, Dr. Peter Ferenci explained in presenting the PEARL-II results at the annual Digestive Disease Week.
PEARL-II was an open-label, 12-week trial which asked the question, what added benefit does daily oral ribavirin provide in conjunction with 3D therapy in noncirrhotic HCV genotype 1b patients previously treated unsuccessfully with a pegylated interferon/ribavirin regimen? The answer, as it turns out, is none.
The 186 participants were randomized to the 3D regimen with or without oral ribavirin. The oral 3D regimen consists of ABT-450 (150 mg)/ritonavir (100 mg)/ombitasvir (25 mg), formulated as a single once-daily pill plus dasabuvir at 250 mg twice daily.
The primary endpoint – a sustained virologic response 12 weeks after completion of the 12-week course of treatment – was achieved in 96.6% of the ribavirin group and 100% who didn’t receive ribavirin. Both rates are superior to the historical sustained virologic response rate seen in studies of the former state-of-the-art regimen of telaprevir plus pegylated interferon and ribavirin, noted Dr. Ferenci of the Medical University of Vienna.
There were no virologic failures in PEARL-II. Two patients stopped the study drugs: one who developed pancreatitis deemed unrelated to treatment and another with anxiety, tachycardia, shortness of breath, and fever considered possibly treatment related.
Results of 3D therapy were equally good in men and women and in former relapsers, nonresponders, and partial responders to pegylated interferon-based regimens.
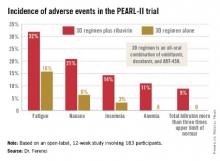
Treatment-related adverse events were more frequent in the ribavirin study arm (see graphic), but were generally mild and manageable.
Asked if in light of the impressive study results he thought physicians should hold off on treating PEARL-II–type patients until the 3D regimen receives marketing approval, Dr. Ferenci replied, "I think if you really need to treat a patient now, you have to use what exists. But I think in half a year your options will change."
PEARL-II was a companion trial to PEARL-III, conducted in previously untreated patients with HCV 1b infection (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1402338]). Both studies were sponsored by AbbVie. Dr. Ferenci reported serving on advisory panels for roughly a dozen pharmaceutical companies.