Vedolizumab, an integrin receptor antagonist, has been approved as a treatment for moderate to severe inflammatory bowel disease in adults who have not had an adequate response to one or more standard treatments, the Food and Drug Administration announced on May 20.
Vedolizumab is administered intravenously, and will be marketed as Entyvio by Takeda Pharmaceuticals America. In trials, vedolizumab was administered intravenously at 0, 2, and 6 weeks, followed by once every 8 weeks for maintenance therapy.
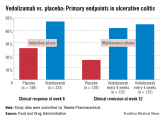
Approval was based on five studies of patients who had not had an adequate response to treatment with corticosteroids, immunomodulators, or tumor necrosis factor blockers. In the two studies of about 900 patients with ulcerative colitis, a higher proportion of patients on vedolizumab achieved and maintained a clinical response and a clinical remission; achieved a corticosteroid-free clinical remission; and "as seen during endoscopy, had improved appearance of the colon," compared with patients on placebo, according to the Food and Drug Administration (FDA).
In the three studies of about 1,500 patients with Crohn’s disease, a greater proportion of those treated with vedolizumab achieved a clinical response, clinical remission, and a corticosteroid-free clinical remission, compared with those on placebo.
Headache, joint pain, nausea, and fever were among the most common adverse events associated with treatment. The most serious events included serious infections; hypersensitivity and infusion-related reactions; and hepatotoxicity.
Because natalizumab (Tysabri), another integrin receptor antagonist, approved for treating multiple sclerosis and Crohn’s disease, has been associated with progressive multifocal leukoencephalopathy (PML), a usually fatal infection, patients in vedolizumab studies were closely followed for PML. Although no cases were reported, "there remains uncertainty regarding the risk of PML in patients taking Entyvio," and the FDA is recommending that health care professionals monitor their patients on vedolizumab for "any new onset, or worsening, of neurological signs and symptoms," according to the FDA statement.
Takeda also will conduct a postmarketing study to evaluate the risk of PML associated with vedolizumab.
At a meeting in December 2013, the FDA’s Gastrointestinal Drugs and Drug Safety and Risk Management advisory committees unanimously supported approval of vedolizumab for Crohn’s disease and ulcerative colitis, agreeing that the benefits of vedolizumab outweighed the potential for progressive multifocal encephalopathy and other possible risks. They recommended that safety be closely monitored after approval, monitoring for potential risks including PML and other infections.
Vedolizumab "blocks the interaction of a specific integrin receptor (expressed on circulating inflammatory cells) with a specific protein (expressed on cells in the interior wall of blood vessels), and thereby blocks the migration of those circulating inflammatory cells across those blood vessels and into areas of inflammation in the gastrointestinal tract," according to the FDA statement announcing the approval.
Serious adverse events associated with vedolizumab should be reported to the FDA at 800-332-1088 or www.fda.gov/MedWatch.
emechcatie@frontlinemedcom.com