CHICAGO – Normal-weight individuals who carry a common variant of the caveolin-1 gene are at nearly fourfold increased risk of the metabolic syndrome compared with those who are homozygous for the major allele, according to a cross-sectional case-control study.
"This particular genotype may improve individual risk profiling and help detect nonobese subjects who are metabolically unhealthy and at increased cardiovascular risk before they experience severe clinical events," Dr. Rene Baudrand observed at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
These findings take on added clinical import in light of mounting evidence that the long-term risks of cardiovascular events and type 2 diabetes in patients with the metabolic syndrome are of similar magnitude regardless of whether an individual is lean, overweight, or obese.
"There is no linear relationship between cardiovascular risk and BMI [body mass index]. So assessment of individual cardiovascular risk should involve more in-depth consideration than BMI alone," said Dr. Baudrand of Brigham and Women’s Hospital, Boston, and the Pontifical Catholic University of Chile, Santiago.
He and others have previously shown that caveolin-1 (cav-1) plays a critical role in cholesterol trafficking and signal transduction. It interacts with insulin receptors, steroid receptors, and ion channels.
The objective of the new study was to investigate the role of a suspected culprit cav-1 gene variant known as cav-1 rs926198 in terms of cardiometabolic risk. Expression of cav-1 has been shown to be significantly diminished in carriers of the variant allele.
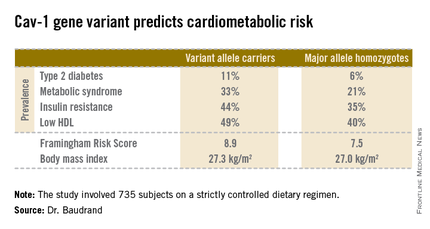
The study included 735 white and Hispanic subjects on a strictly controlled dietary regimen. They averaged 45 years of age, with a mean BMI of 27.2 kg/m2. Two-thirds of participants were hypertensive, 9% had type 2 diabetes, and 30% had metabolic syndrome. Among the 200 obese subjects, the prevalence of metabolic syndrome was 62%, compared with 18% among the 535 nonobese subjects.
Fully 57% of subjects were found to carry the cav-1 gene variant. The other 43% were homozygous for what is known as the major allele. Carriers of the cav-1 gene variant were found to have significantly higher rates of type 2 diabetes, prediabetes, metabolic syndrome, and other abnormalities than those with the major allele (see chart). These differences were not explainable by differences in BMI; indeed, the average BMI in the two groups was nearly identical, according to Dr. Baudrand.
He and his coworkers also sought to learn whether study participants with metabolic syndrome would be more likely to have a sibling with metabolic syndrome if they were concordant for the cav-1 gene variant. This proved to be the case. In evaluating 348 subjects belonging to 142 sibships, the likelihood of concordance for metabolic syndrome was threefold greater if the siblings shared the cav-1 risk allele than if they were cav-1 discordant.
Upon closer scrutiny of the data, it became apparent that the seriously unfavorable cardiometabolic profile associated with the cav-1 gene variant was confined to the nonobese subjects. In a multivariate analysis adjusted for age, gender, BMI, and study site, the likelihood of metabolic syndrome among nonobese subjects with the variant allele was 3.86-fold higher than in nonobese individuals carrying the major allele. In addition, the adjusted likelihood of type 2 diabetes was 2.26-fold greater and that of low HDL was 1.78-fold greater, with all differences highly statistically significant. In contrast, the risk of these cardiometabolic problems in obese subjects didn’t vary regardless of the form of the cav-1 gene they possessed.
On the basis of these encouraging findings, the next step will be to conduct prospective studies to see if cav-1 genotyping is useful as a screening tool for cardiometabolic risk in nonobese patients. Further investigation is also warranted to identify the specific mechanisms by which the cav-1 risk allele results in cardiometabolic impairment. Among the leading possibilities are defects in aldosterone signaling, the insulin receptor, or adipocyte function, Dr. Baudrand said.
This study was supported by the National Institutes of Health and the Chilean National Science and Technology Research Fund. Dr. Baudrand reported having no financial conflicts.