1. American Cancer Society. Cancer facts & figures 2012. Accessed 2 May 2013 at www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012.
2. National Cancer Institute. SEER Stat Fact Sheets: Bladder. Accessed 14 December 2012 at seer.cancer.gov/statfacts/html/urinb.html.
3. Lynch CF, Davila JA, Platz CE. Cancer of the urinary bladder. In: Ries LAG, Young JL, Keel GE, et al, editors. SEER survival monograph: cancer survival among adults: US SEER program 1988-2001, patient and tumor characteristics. NIH Pub. No. 07-6215. Bethesda (MD): National Cancer Institute; 2007:181–92.
4. Brennan P, Bogillot O, Cordier S, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer 2000;86:289–94.
5. Castelao JE, Yuan JM, Skipper PL, et al. Gender and smoking-related bladder cancer risk. J Natl Cancer Inst 2001;93:538–45.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, volume 83. Tobacco smoke and involuntary smoking. Lyon, France: World Health Organization; 2004. Accessed 2 May 2013 at http://monographs.iarc.fr/ENG/Monographs/vol83/index.php.
8. Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer, BJU Int 2007;100:281–6.
9. Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urology 2007;25:285–95.
10. Piper JM, Tonascia J, Matanoski GM. Heavy phenacetin use and bladder cancer in women aged 20 to 49 years. N Engl J Med 1985;313:292–5.
11. Knight A, Askling J, Granath F, et al. Urinary bladder cancer in Wegener’s granulomatosis: risks and relation to cyclophosphamide. Ann Rheum Dis 2004;63:1307–11.
12. Fairchild WV, Spence CR, Solomon HD, Gangai MP. The incidence of bladder cancer after cyclophosphamide therapy. J Urology 1979; 122:163.
13. Brock N. The development of mesna for the inhibition of urotoxic side effects of cyclophosphamide, ifosfamide, and other oxazaphosphorine cytostatics. Recent Results Cancer Res 1980;74:270–8.
14. Spruck CH, Ohneseit PF, Gonzalez-Zulueta M, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res 1994;54:784–8.
15. Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res 2003;63:8108–12.
16. Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 1994;331:1259–64.
17. Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol 2006;24:5552–64.
18. National Cancer Institute. Bladder and other urothelial cancers screening (PDQ). January 23, 2012. Accessed 14 December 2012 at www.cancer.gov/cancertopics/pdq/screening/bladder/HealthProfessional.
19. Farrow GM, Utz DC, Rife CC, Greene LF. Clinical observations on sixty-nine cases of in situ carcinoma of the urinary bladder. Cancer Res 1977;37:2794–8.
20. Davis R, Jones J, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 2012;188(6 Suppl):2473–81.
21. Silverman SG, Leyendecker JR, Amis ES Jr. What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology 2009;250:309–23.
22. Sadow CA, Silverman SG, O’Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an academic medical center. Radiology 2008;249:195–202.
23. Murphy WM, Soloway MS, Jukkola AF, et al. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer 1984;53:1555–65.
24. Halachmi S, Linn JF, Amiel GE, et al. Urine cytology, tumour markers and bladder cancer. Br J Urol 1998;82:647–54.
25. Koss LG, Deitch D, Ramanathan R, Sherman AB. Diagnostic value of cytology of voided urine. Acta Cytol 1985;29:810–6.
26. Yafi FA, Brimo F, Auger M, et al. Is the performance of urinary cytology as high as reported historically? A contemporary analysis in the detection and surveillance of bladder cancer. Urol Oncol 11 Feb 2013. [Epub ahead of print]
27. van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol 2005;47:736–48.
28. Vrooman OPJ, Witjes JA. Urinary markers in bladder cancer. Eur Urol 2008;53:909–16.
29. Toma MI, Friedrich MG, Hautmann SH, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol 2004;22:145–9.
30. Jones JS. DNA–based molecular cytology for bladder cancer surveillance. Urology 2006;67(3 Suppl 1):35–45.
31. Glas AS, Roos D, Deutekom M, et al. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol 2003;169:1975–82.
32. Sharma S, Zippe CD, Pandrangi L, et al. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol 1999;162:53–7.
33. Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol 2007;178:68–73.
34. Grossman HB, Gomella L, Fradet Y, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol 2007;178:62–7.
35. Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol 2004;171:135–8.
36. Denzinger S, Burger M, Walter B, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007;69:675–9.
37. Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol 2010;184:
38. Cauberg EC, Mamoulakis C, de la Rosette JJ, de Reijke TM. Narrow band imaging-assisted transurethral resection for non-muscle invasive bladder cancer significantly reduces residual tumour rate. World J Urol 2011;29:503–9.
39. Cauberg EC, Kloen S, Visser M, et al. Narrow band imaging cystoscopy improves the detection of non–muscle-invasive bladder cancer. Urology 2010;76:658–63.
40. Herr HW, Donat SM. Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy. Br J Urol Intl 211;107:396–8.
41. Zaak D, Karl A, Knüchel R, et al. Diagnosis of urothelial carcinoma of the bladder using fluorescence endoscopy. Br J Urol Intl 2005;96:217–22.
42. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178:2314–30.
43. Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 1999;162:74–6.
44. Hollenbeck BK, Miller DC, Taub D, et al. Risk factors for adverse outcomes after transurethral resection of bladder tumors. Cancer 2006;106:1527–35.
45. Nieder AM, Meinbach DS, Kim SS, Soloway MS. Transurethral bladder tumor resection: intraoperative and postoperative complications in a residency setting. J Urol 2005;174:2307–9.
46. Traxer O, Pasqui F, Gattegno B, Pearle MS. Technique and complications of transurethral surgery for bladder tumours. Br J Urol Intl 2004;94:492–6.
47. Mydlo JH, Weinstein R, Shah S, et al. Long-term consequences from bladder perforation and/or violation in the presence of transitional cell carcinoma: results of a small series and a review of the literature. J Urol 1999;161:1128–32.
48. Frachet O, Cordier G, Henry N, et al. Bladder perforation during transurethral resection of bladder tumour: a review. Prog Urol 2007;17:1310–2.
49. Golan S, Baniel J, Lask D, et al. Transurethral resection of bladder tumour complicated by perforation requiring open surgical repair - clinical characteristics and oncological outcomes. Br J Urol Intl 2011; 107:1065–8.
50. Kihl B, Nilson AE, Pettersson S. Thigh adductor contraction during transurethral resection of bladder tumours: evaluation of inactive electrode placement and obturator nerve topography. Scand J Urol Nephrol 1981;15:121–5.
51. Del Rosso A, Pace G, Masciovecchio S, et al. Plasmakinetic bipolar versus monopolar transurethral resection of non-muscle invasive bladder cancer: a single center randomized controlled trial. Intl J Urol 2013;20:399–403.
52. Puppo P, Bertolotto F, Introini C, et al. Bipolar transurethral resection in saline (TURis): outcome and complication rates after the first 1000 cases. J Endourol 2009;23:1145–9.
53. Kitamura T, Mori Y, Ohno N, et al. Case of bladder perforation due to the obturator nerve reflex during transurethral resection (TUR) of bladder tumor using the TUR in saline (Turis) system under spinal anesthesia [in Japanese]. Masui 2010;59:386–9.
54. American Joint Committee on Cancer.: Urinary bladder. In: Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010:497–505.
55. Elbe J, Sauter G, Epstein J, Sesterhenn I. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary and male genital organs. Lyon, France: IARC Press;2004.
56. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 2000;164:680–4.
57. Böhle A, Brandau S. Immune mechanisms in bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. J Urol 2003;170964–9.
58. Kawai K, Miyazaki J, Joraku A, et al. Bacillus Calmette-Guérin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci 2013;104:22–7.
59. Takeuchi A, Dejima T, Yamada H, et al. IL-17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guérin treatment against bladder cancer. Eur J Immunol 2011;41:246–51.
60. Gopal R, Lin Y, Obermajer N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 2012;42:364–73.
61. Suttmann H, Jacobsen M, Reiss K, et al. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol 2004;172:1490–5.
62. Luo Y, Knudson MJ. Mycobacterium bovis bacillus Calmette-Guérin-induced macrophage cytotoxicity against bladder cancer cells. Clin Dev Immunol 2010;2010:357591.
63. Rischmann P, Desgrandchamps F, Malavaud B, Chopin DK. BCG intravesical instillations: recommendations for side-effects management. Eur Urol 2000;37(Suppl 1):33–6.
64. Luo Y, Chen X, Downs TM, et al. IFN-α 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guérin immunotherapy. J Immnuol 1999;162:2399–2405.
65. Joudi FN, Smith BJ, O’Donnell MA. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon α-2B for reducing recurrence of superficial bladder cancer. Urol Oncol 2006;24:344–8.
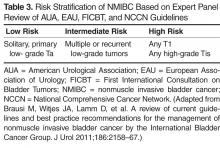
66. Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 2011;186:2158–67.
67. Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 2004;171:2186–90.
68. Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol 2006;175:1641–4.
69. Oddens JR, Van der Meijden AP, Sylvester R. One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? Eur Urol 2004;46:336–8.
70. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9.
71. Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013;63:462–72.
72. Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003;169:90–5.
73. Sylvester RJ, van der Meijden AP, Witjes JA, Kurth J. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 2005;174:86–91.
74. Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247–56.
75. Jones G, Cleves A, Wilt TJ, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev 2012;CD009294.
76. Kurth H, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer 1995;31A:1840–6.
77. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:465–66.
78. Rodríguez Faba O, Palou J. Predictive factors for recurrence progression and cancer specific survival in high-risk bladder cancer. Curr Opin Urol 2012;22:415–20.
79. Streeper NM, Simons CM, Konety BR, et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. Br J Urol Intl 2009;103:475–9.
80. Witjes JA. Prognosis of T1G3 bladder cancer: how well can we predict progression? Eur Urol 2012; 62:126–7.
81. Khochikar M. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. Br J Urol Intl 2011;108(Pt 2):E288–9.
82. De Berardinis E, Busetto GM, Antonini G, et al. T1G3 high-risk NMIBC (non-muscle invasive bladder cancer): conservative treatment versus immediate cystectomy. Intl Urol Nephrol 2011;43:1047–57.
83. Badalato GM, Gaya JM, Hruby G, et al. Immediate radical cystectomy vs conservative management for high grade cT1 bladder cancer: is there a survival difference? Br J Urol Intl 2012;110:1471–7.
84. Sternberg IA, Keren Paz GE, Chen LY, et al. Role of immediate radical cystectomy in the treatment of patients with residual T1 bladder cancer on restaging transurethral resection. BJU Intl 2012;112:54–9.
85. Canter D, Egleston B, Wong YN, et al. Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: A SEER database analysis. Urol Oncol 2013;31:866–70.
86. Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol 2009;27:347–51.
87. Heney NM, Nocks BN, Daly JJ, et al. Ta and T1 Bladder cancer: location, recurrence and progression. Br J Urol 2008;54:152–7.
88. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines): Bladder cancer. Jenkintown (PA): NCCN; 2012.
89. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1 and Tis): a 2007 update. J Urol 2007;178:2314–30.
90. van den Bosch S, Witjes JA. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol 2011;60:493–500.
91. Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol 2001;166:1296–9.
92. Jäger W, Thomas C, Haag S, et al. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. BJU Int; 2011;108(Pt 2):E284–8.
93. Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 2012 May 9. [Epub ahead of print]
94. Yates DR, Rouprêt M. Contemporary management of patients with high-risk non-muscle-invasive bladder cancer who fail intravesical BCG therapy. World J Urol 2011;29:415–22.