• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
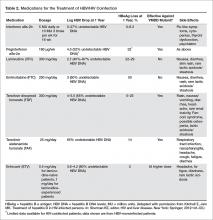
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.