On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
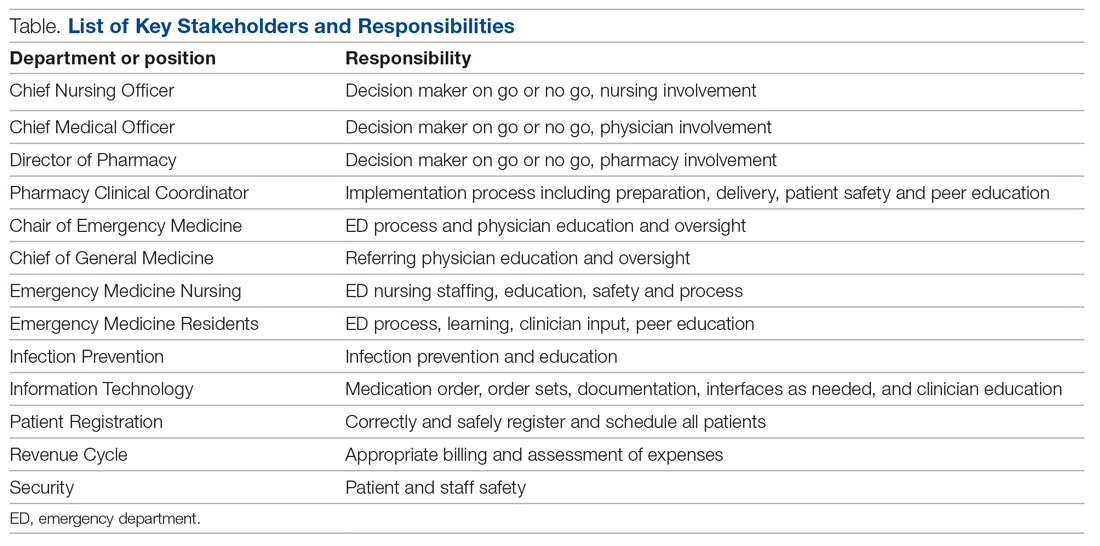
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
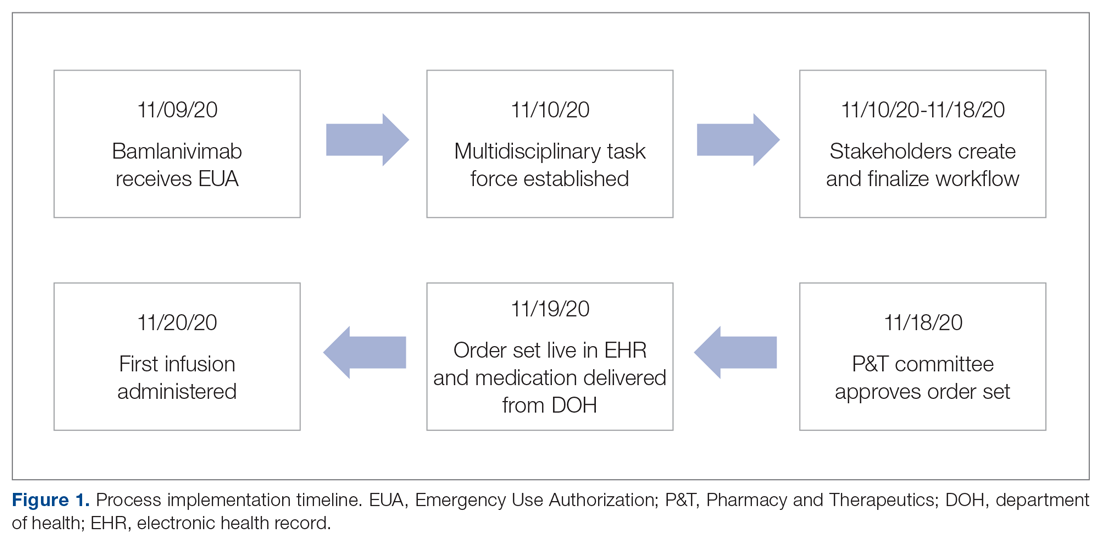
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.