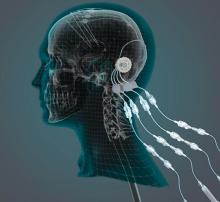
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”