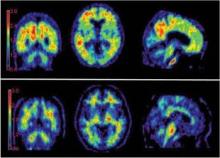
The imaging agent florbetapir F 18 injection (Amyvid), used to exclude a diagnosis of Alzheimer’s disease, is being evaluated for Medicare reimbursement, the Centers for Medicare and Medicaid Services has announced.
The agency stated on Oct. 9 that it expected a preliminary decision by July 2013, and a final decision by October 2013.
The CMS is accepting public comments on its proposed coverage of florbetapir until Nov. 8.
Florbetapir, approved by the Food and Drug Administration in April, is used in conjunction with positron emission tomography (PET). Eli Lilly, which owns florbetapir maker Avid Radiopharmaceuticals, requested the coverage determination. The company laid out its case for reimbursement in a 47-page letter to the CMS in June.
In the request, Lilly noted that the CMS approved coverage of FDG-PET for patients who had a recent dementia diagnosis and cognitive decline over 6 months and who met diagnostic criteria for Alzheimer’s disease and frontotemporal dementia.
According to Avid, florbetapir binds to beta-amyloid plaques in the brain, which are considered markers for Alzheimer’s. Without the ability to see those plaques in real time, diagnosis has been based on symptoms and cognitive testing. But that is imperfect, missing perhaps a fifth of cases.
At the time of florbetapir’s approval, the Alzheimer's Association said the imaging agent would be useful in diagnosis and in tracking of treatment response, but also expressed concern about potentially unscrupulous uses of the technology.
The organization has convened a task force with the Society of Nuclear Medicine to develop guidelines on florbetapir use.
An estimated 5 million Americans have Alzheimer’s, and the incidence is rising with the aging of the U.S. population. Only 4% of those with the disease are under the Medicare age of 65, according to Lilly; 40%-45% of Americans over age 75 are estimated to have the disease.