News
Priorities for determining the etiology of incontinence
Letters from readers
Marjorie L. Pilkinton, MD; Dara Shalom, MD; and Harvey A. Winkler, MD



Dr. Winkler reports that he is a consultant to Astora Women’s Health, Boston Scientific, and Kimberly-Clark. Drs. Pilkinton and Shalom report no financial relationships relevant to this article.

As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
Letters from readers
These surgeons review indications and demonstrate patient positioning and their minimally invasive fascia lata harvest technique.

Yes: Median episiotomy was associated with an increased rate of 3rd- and 4th-degree perineal laceration in both nulliparous and multiparous women...
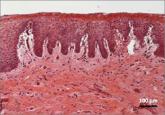
With more than 1 billion menopausal women likely to be affected by vulvovaginal atrophy worldwide by 2025, the need for effective remedies is...
No, provided the patient undergoes careful office evaluation instead, according to this systematic review and meta-analysis Rachaneni S, Latthe P...
