Expert Commentary

What insect repellents are safe during pregnancy?
Physicians have requested information about which insect repellents to recommend to help pregnant women avoid Zika and other mosquito-born...

Plus more Letters to the Editor topics
Picaridin is not mentioned in this brief report from Drs. Chelliah and Duff. I suggest reviewing the July 2015 Consumer Reports article on repellents; picaridin is a likely safer alternative to DEET, with the highest efficacy of all those tested, at least in Sawyer Fisherman’s Formula Picaridin Insect Repellent and Natrapel 8 Hour Insect Repellent. Products that have little or no efficacy also were not mentioned, including Avon Skin So Soft, Coleman Naturals Insect Repellent Snap Band, and SuperBand Wristband. In addition, the concentration of products is very important, as is the precise formulation within brands. For example, Off! Deep Woods VIII (with DEET 25%) is very effective versus Off! FamilyCare II Clean Feel (with picaridin 5%), which has very little benefit.
David H. Janowitz, MD
Houston, Texas
In our short discussion of mosquito repellents, we based our recommendations on publications from the Centers for Disease Control and Prevention (CDC) and the Florida Department of Health. Those publications presented DEET (N,N-diethyl-m-toluamide) at the top of the list for preferred repellents. A recent publication from the Organization of Teratology Information Specialists (MotherToBaby, September 2013) indicated that, in a concentration of 20% to 30%, DEET was safe in pregnancy and was effective in protecting against 90% of all mosquito bites and tick attachments. Increasing the concentration of DEET above 30% does not enhance the product’s effectiveness or prolong its duration of action.
However, Dr. Janowitz is correct in stating that other agents are also highly effective and safe in pregnancy. These agents include picaridin (20%) and oil of lemon/eucalyptus (30%). We thank Dr. Janowitz for directing us to the most recent testing program conducted by Consumer Reports.1 That testing program demonstrated that Sawyer Fisherman’s Formula Picaridin and Natrapel 8 Hour, which each contain 20% picaridin, and Off! Deep Woods VIII, which contains 25% DEET, kept Aedes mosquitoes from biting for approximately 8 hours. The Sawyer product was also effective in preventing bites from the Culex mosquitoes, which carry West Nile virus, and deer ticks, which can transmit Lyme disease. Repel Lemon Eucalyptus (30%) stopped Aedes mosquito bites for 7 hours.
In the Consumer Reports testing program, IR3535 products, which we recommended in our article, did not perform well, nor did repellents that contained only 7% DEET or less than 20% picaridin. Moreover, products made from natural plant oils—such as citronella, lemongrass oil, cedar oil, geraniol, rosemary oil, and cinnamon oil—were not particularly effective. Some did not last for more than 1 hour; some failed immediately.
When applying any of these products, individuals should observe the following guidelines:
Reference
While I share the optimism Dr. Advincula expressed in his recent guest editorial regarding a change in the direction of the pendulum that swung away from use of the power morcellator, I feel compelled to express the opinion that this entire fiasco has been nothing other than an outrageous regulatory overreach.
Shortly after the US Food and Drug Administration (FDA) issued its proclamation in April 2014, the Society of Gynecologic Oncology repudiated the bogus statistics that were being used to describe the incidence of leiomyosarcoma and, further, stated that it would not matter how someone’s uterus containing this rare tumor was removed because the outcome would be poor. Similarly, the American Journal of Obstetrics and Gynecology published an article enumerating the expected significant increase in complications and the resulting misery that could be expected for patients whose management was diverted from minimally invasive to open hysterectomy.1 The AAGL also expressed opinions that this was an unnecessary, and counterproductive, policy—all to no avail.
My optimism, however, is tempered by a number of questions: 1) Why did it take more than a year for 36 nationally recognized gynecologic surgeons to write a letter to the FDA denouncing the warning, yet again, and reiterating the errors in analysis used to establish the policy? 2) Why are gynecologic surgeons only now being asked to serve in the FDA’s Network of Experts? Should not that have been the case before the warning was issued? 3) If the perioperative outcomes are similar using a containment bag compared with open morcellation, what is the benefit of using the containment system? I, for one, think that prolonging a procedure another half hour is significant.

Physicians have requested information about which insect repellents to recommend to help pregnant women avoid Zika and other mosquito-born...

With practical, evidence-based, sound clinical judgement, I believe that it can
Zika virus infection, although typically mild and often asymptomatic, can have serious consequences in pregnancy. As the pandemic rapidly spreads...
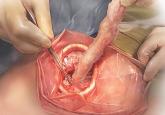
Use beef tongue, a cardboard box, and a homemade self-retaining wound retractor to teach or perfect your extracorporeal C-incision tissue...
