New clinical practice guideline advises neoadjuvant chemotherapy for certain women with ovarian cancer
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-3473.
It has long been held as a central dogma that primary cytoreductive surgery (PCS) is the preferred initial treatment for women with newly diagnosed ovarian cancer.8 However, PCS is associated with substantial morbidity, and the ability to achieve optimal cytoreduction (<1 cm of residual disease), an important prognostic factor, is often compromised in women with significant tumor burden.9,10
Neoadjuvant chemotherapy, in which chemotherapy is administered prior to surgical cytoreduction, challenges the traditional treatment paradigm for advanced-stage ovarian cancer. Several randomized controlled trials have reported equivalent survival for primary surgical cytoreduction and NACT. Importantly, women who received NACT had fewer complications and were more likely to have optimal cytoreduction at the time of surgery.11,12 These studies have limitations, however, and the role of NACT remains uncertain.
To help guide clinicians, the Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an expert panel to provide recommendations and guidance on the evaluation of women for and the use of NACT in the setting of advanced ovarian cancer.13
Related article:
2015 Update on cancer
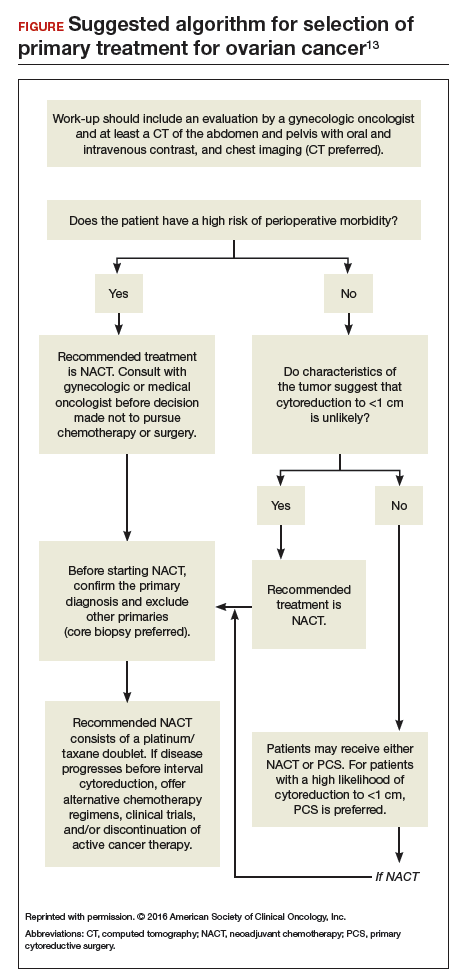
Recommendation: Clinical evaluation and patient selection
Strong clinical evidence supports that all women with suspected stage IIIC or stage IV ovarian cancer should be evaluated by a gynecologic oncologist prior to the initiation of therapy. The evaluation should include at least a computed tomography scan of the chest, abdomen, and pelvis to assess the extent of disease and resectability. A preoperative risk assessment should be performed to assess risk factors for increased morbidity and mortality.
Women who have a high perioperative risk profile or a low likelihood of achieving cytoreduction to 1 cm or less of residual tumor should receive NACT. Prior to the initiation of NACT, histologic confirmation of ovarian cancer should be obtained.13
Outcomes for neoadjuvant chemotherapy versus primary cytoreduction
Four phase 3 randomized controlled trials (EORTC 55971, CHORUS, JCOG0602, and SCORPION) suggest that NACT is noninferior to PCS with regard to progression-free survival and overall survival. NACT is associated with less perioperative and postoperative morbidity and mortality and is associated with shorter hospital stays.
To date, complete data are available only from the EORTC and CHORUS trials, which both demonstrated similar progression-free survival and overall survival for NACT and PCS. Critics have noted, however, that both trials have shorter median overall survival for the PCS groups than were previously reported in other phase 3 studies in the United States, suggesting the possibility of different patient populations or less aggressive "surgical effort." Thus, PCS remains the preferred management strategy for women with advanced-stage ovarian cancer in whom there is a high likelihood of optimal cytoreduction.13
Recommendation: Use of neoadjuvant chemotherapy
Patients who are appropriate candidates for NACT should be treated with a platinum and taxane doublet and should receive interval cytoreduction following 3 to 4 cycles of therapy if a favorable response is noted. Patients whose disease progresses despite NACT have a poor prognosis, and there is little role for surgical treatment with the exception of palliative purposes.13
Neoadjuvant chemotherapy is a noninferior and appropriate treatment option for women who are poor surgical candidates or who have a low likelihood of optimal cytoreduction. When optimal cytoreduction is possible, however, PCS is preferred (see FIGURE). The data on the efficacy of NACT for ovarian cancer have led to increased use of this treatment in the United States.