Long-acting reversible contraception (LARC) use continues to increase in the United States. According to the most recent estimates from 2014, 14% of women use either an intrauterine device (IUD) or the etonogestrel implant.1 Forms of LARC currently available in the United States include:
- 4 hormone-releasing IUDs
- 1 nonhormonal copper IUD, and
- 1 hormonal subdermal implant.
The hormone-releasing IUDs all contain levonorgestrel (LNG). These include two 52-mg LNG products and a 19.5-mg LNG IUD, which are currently approved by the US Food and Drug Administration (FDA) for contraception for 5 continuous years of use. In addition, a 13.5-mg LNG IUD is FDA-approved for 3 years of use. The hormonal subdermal implant, which contains etonogestrel, is FDA-approved for 3 years of use. Although major complications with IUDs (perforation, expulsion, intrauterine infection)and implants (subfascial implantation, distant migration) are rare, adverse effects that can affect continuation—such as irregular bleeding—are more common.2,3
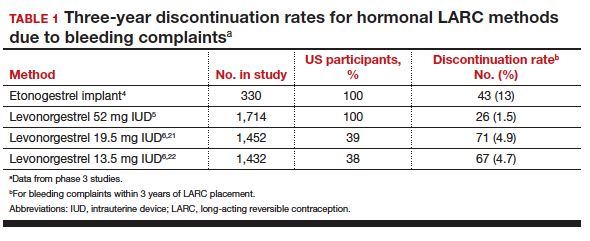
Contraceptive discontinuation due to bleeding concerns occurs more frequently with the etonogestrel implant than with LNG IUDs (TABLE 1). In a large prospective study in the United States, 13% of women discontinued the implant during 3 years of follow-up due to bleeding pattern changes.4 In comparison, the 3-year discontinuation rate for bleeding complaints with the 52-mg LNG IUD is 1.5%.5 The 3-year discontinuation rate is higher with the 19.5-mg and 13.5-mg LNG IUDs (4.9% and 4.7%, respectively).6 The discontinuation rate for bleeding complaints within 5 years of use remains higher for the 19.5-mg LNG IUD (5.2%) compared with the 52-mg LNG IUD (2.2%).7,8
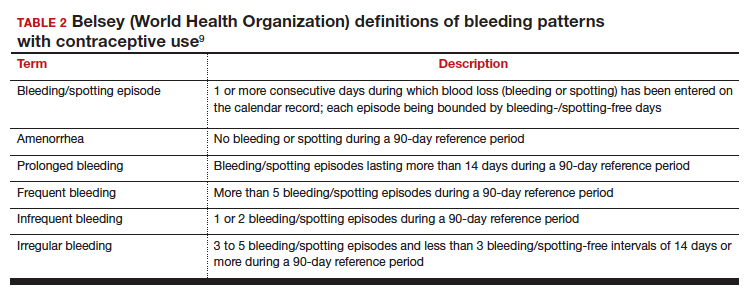
Notably, it is important to use standardized definitions to understand and compare bleeding concerns with LARC use. The Belsey criteria of the World Health Organization (WHO), a standard used for decades, describe bleeding patterns using 90-day reference periods or intervals (TABLE 2).9 Bleeding patterns that decrease flow (amenorrhea, infrequent bleeding) often are considered favorable, and those that increase bleeding or irregularity often are considered unfavorable. These criteria are commonly used in package labeling to describe bleeding patterns with extended use.
In this Update, we examine recent data evaluating differences in bleeding patterns with the 3 doses of the LNG IUD, predictors of abnormal bleeding with the etonogestrel implant, and the impact of timing on postpartum etonogestrel implant placement.
Continue to: Bleeding patterns with progestin-containing IUDs vary according to the LNG dose...