Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
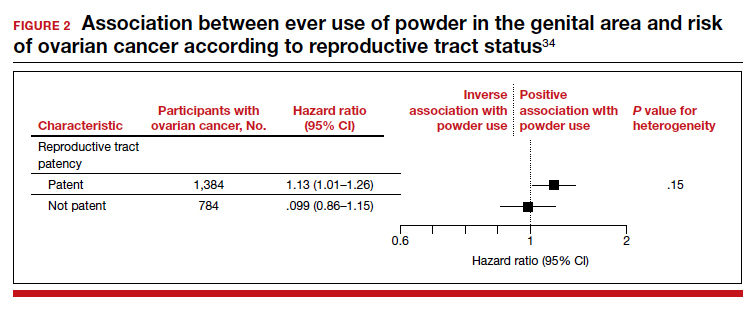
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●
The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.