Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.
 Courtesy Dr. Shifa Turan
Courtesy Dr. Shifa Turan
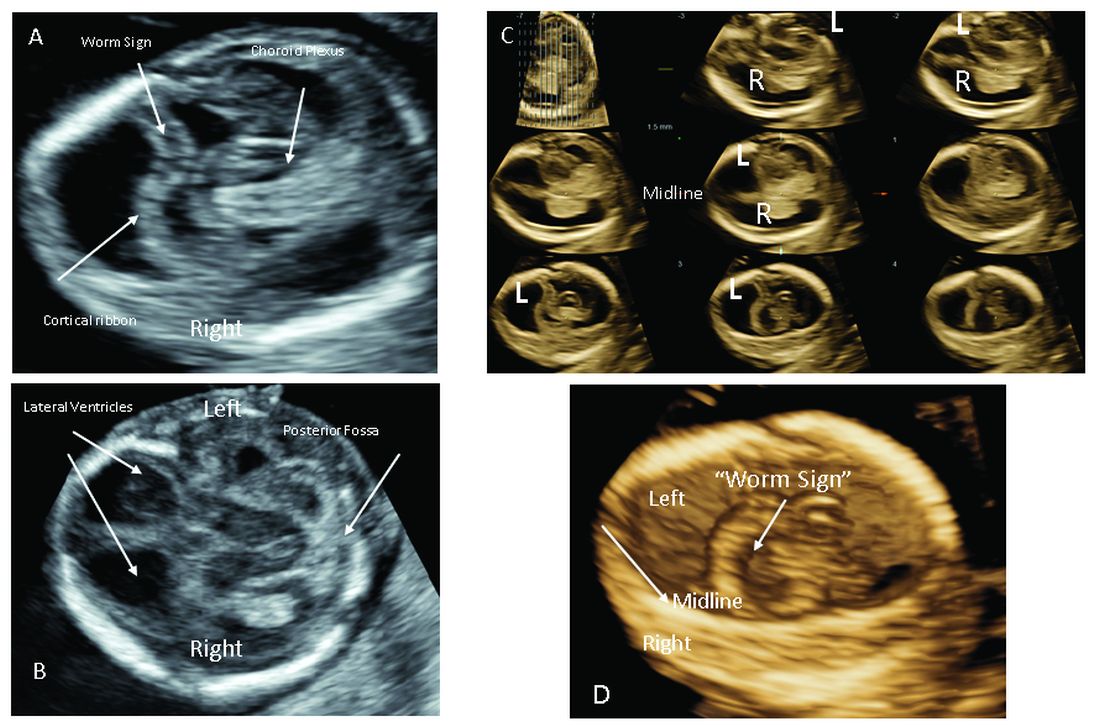
Figure 1/Cortical disruption. First-trimester ultrasound at 13 + 5 weeks’ gestation with early signs of cortical disruption. A) Bilayer structures noted floating freely in cerebrospinal fluid spaces, detached from the convexity of the skull. B) Infratentorial axial image shows equal anterior ventricles, preserved midline, and posterior fossa development. C) 3D-tomographic ultrasound imaging with adjusting slice of 1.5 mm localizes cortical disruption as left supratentorial. D) 3-D rendering reveals disruption confined to the left cerebrum. Noninvasive prenatal testing was performed and showed low risk for trisomy 21, 13, 18 and Turner syndrome. Invasive testing was declined. In genetic testing performed after birth, the baby was found to have a duplication on chromosome Xq12 (which includes 6 cataloged genes: AR, MSN, MIR223, VSIG4, EDA2R, and HEPH) – a variation of unknown significance.
Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.

